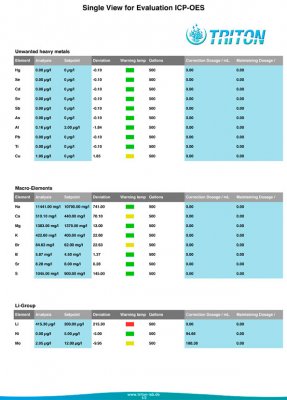
Just got my results in today. Not too bad I suppose considering some I've read. Not too happy with the Copper result considering I've had this issue for a few years and I still cannot find the source. Anyway, some specifics:
Tank: 500g 'SPS' dominant reef. Barebottom. Old School Berlin System. Component of a 650g system which includes a 40g 'LPS' tank and a common 125g sump.
Age: Approx: 1 year since last rebuild. 50% BRS Pukani Rock, 45%, Premium Aquatics 'Mandano', 5% 15 year old Live Rock.
Salt: Reef Crystals.
Additives & Supplements: Kalkwasser, GAC & GFO, Very occasional hits of Acropower.
Others: Temp: 77.6 - 79.0, Salinity 35 ppt. pH: 8.1 - 8.3 (Pinpoint Monitor)
Any comments or questions are welcomed and appreciated.
Sorry if I uploaded this incorrectly.
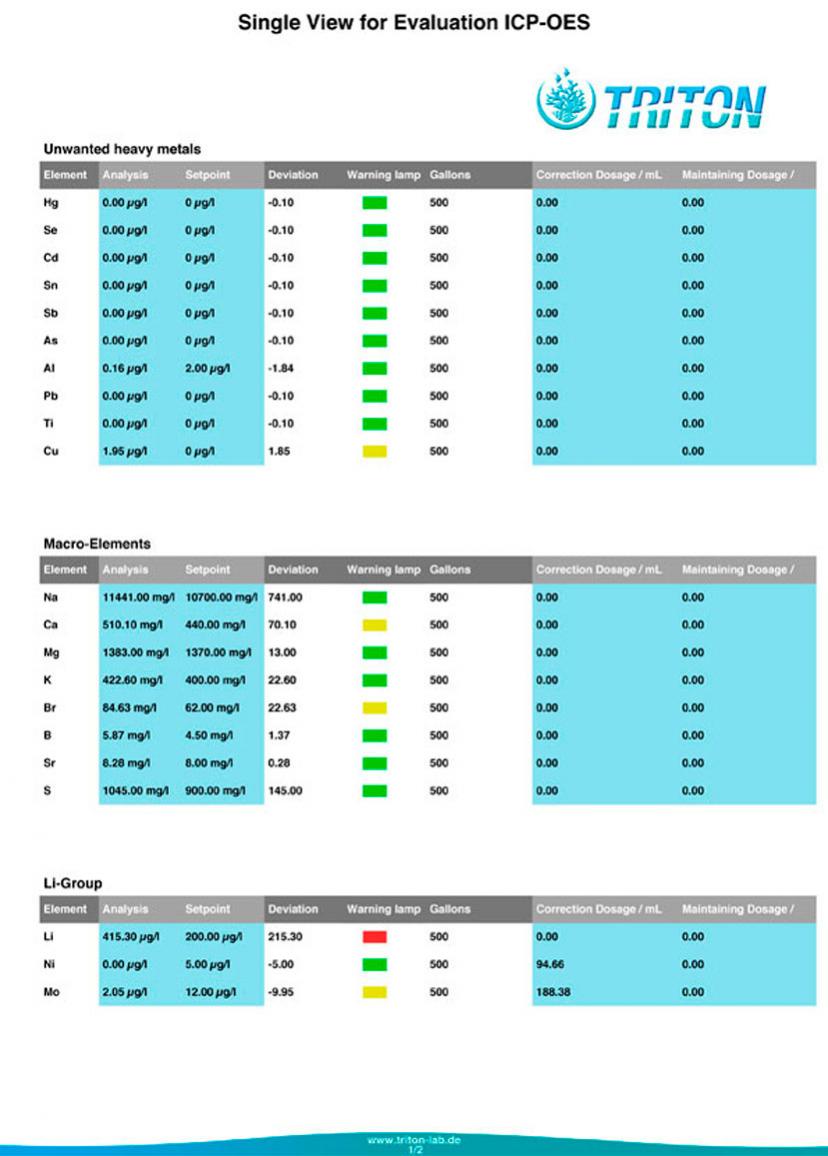
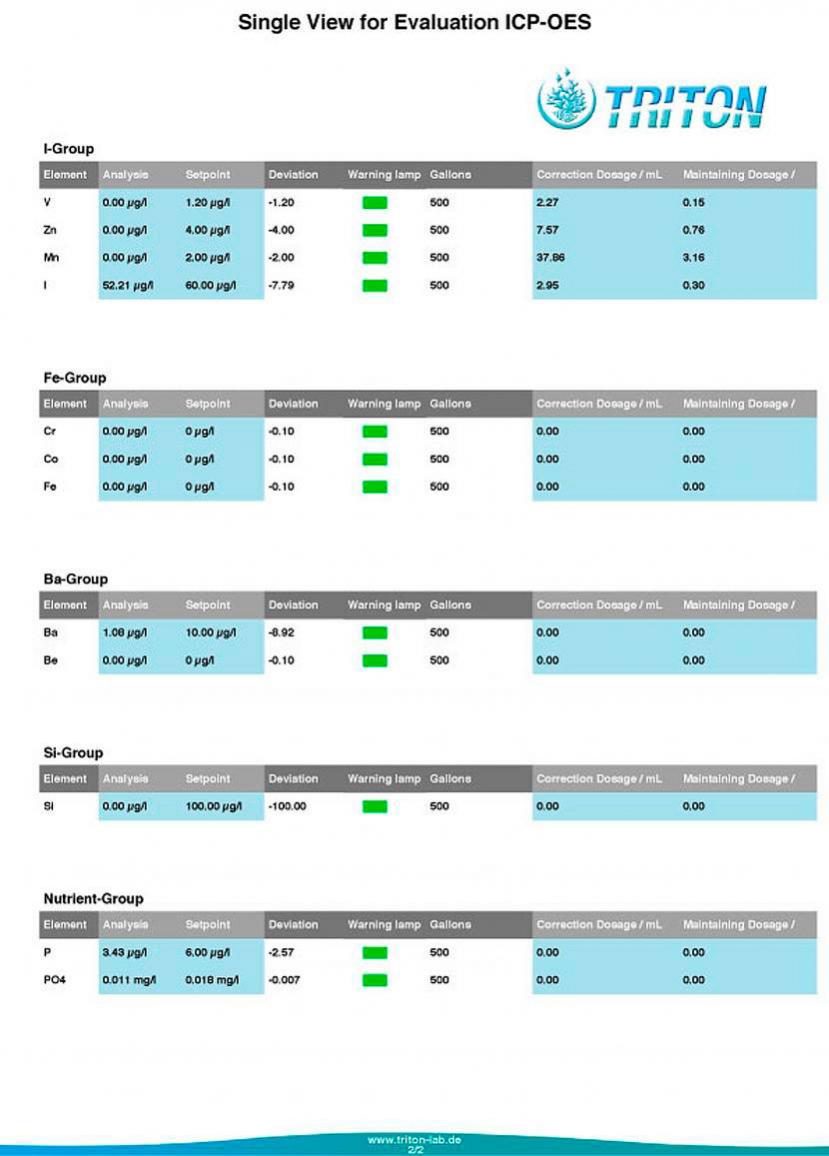
Tank: 500g 'SPS' dominant reef. Barebottom. Old School Berlin System. Component of a 650g system which includes a 40g 'LPS' tank and a common 125g sump.
Age: Approx: 1 year since last rebuild. 50% BRS Pukani Rock, 45%, Premium Aquatics 'Mandano', 5% 15 year old Live Rock.
Salt: Reef Crystals.
Additives & Supplements: Kalkwasser, GAC & GFO, Very occasional hits of Acropower.
Others: Temp: 77.6 - 79.0, Salinity 35 ppt. pH: 8.1 - 8.3 (Pinpoint Monitor)
Any comments or questions are welcomed and appreciated.
Sorry if I uploaded this incorrectly.