Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,252
- Reaction score
- 63,599
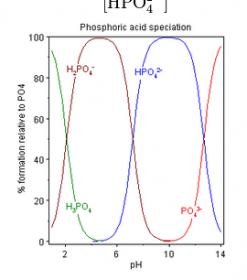
Phosphate has numerous forms that exist in reef aquaria, and the relative proportion of the various forms depends on several factors, especially pH. The actual forms present have a big impact on various processes, such as phosphate binding to calcium carbonate surfaces.
In seawater at pH 8.2, which form of inorganic phosphate predominates?
A. H3PO4
B. H[SIZE=-1]2[/SIZE]PO[SIZE=-1]4-[/SIZE]
C. HPO4--
D. PO[SIZE=-1]4---[/SIZE]
I'd rate this one as medium-high difficulty, except that everyone has at least a 25% chance of success.
Not that it helps any for this question, but my avatar is a phosphate molecule. Specifically, since the double bond is shown, it is H3PO4 without any hydrogen atoms shown. If it were really PO4---, all four phosphate atoms would be identical, with none able to be specified as the one with the double bond to the central phosphorus atom.
Good luck.
In seawater at pH 8.2, which form of inorganic phosphate predominates?
A. H3PO4
B. H[SIZE=-1]2[/SIZE]PO[SIZE=-1]4-[/SIZE]
C. HPO4--
D. PO[SIZE=-1]4---[/SIZE]
I'd rate this one as medium-high difficulty, except that everyone has at least a 25% chance of success.
Not that it helps any for this question, but my avatar is a phosphate molecule. Specifically, since the double bond is shown, it is H3PO4 without any hydrogen atoms shown. If it were really PO4---, all four phosphate atoms would be identical, with none able to be specified as the one with the double bond to the central phosphorus atom.
Good luck.
Last edited: