Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,142
- Reaction score
- 63,494
Reef Chemistry Question of the Day #92
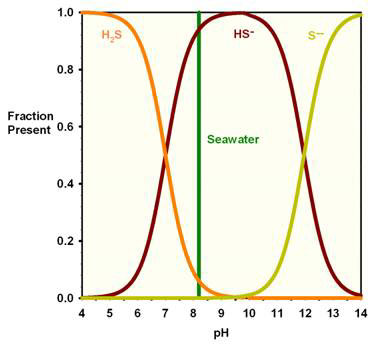
Hydrogen sulfide is most likely present in which of the following forms in seawater at pH 8.2?
A. H2S
B. HS-
C. HS+
D. S--
Good luck!
.
Hydrogen sulfide is most likely present in which of the following forms in seawater at pH 8.2?
A. H2S
B. HS-
C. HS+
D. S--
Good luck!
.