Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
25 year old 75G Jaubert Plenum on top with 30G EcoSystem Mud/Macro
- Build Thread
- Thread starter Subsea
- Start date
- Tagged users None
I'm all up for trying that as well. Another thing is if the pH gets low enough to dissolve some Ca, Mg etc from the coral gravel, you got a calcium reactor. I have no idea if that's possible, maybe that requires an even lower pH.How about co2 injection to maintain an anoxic zone for denitrification.
I've tested water before and after our calcium reactors, just to see if the NO3 is consumed in there, but didn't show any differences.
How to dissolve the CO2? A bubble a guess would just go through the sand up to the surface.
Dyminco inject co2 straight into the substrate, computor controlled via the orp probeI'm all up for trying that as well. Another thing is if the pH gets low enough to dissolve some Ca, Mg etc from the coral gravel, you got a calcium reactor. I have no idea if that's possible, maybe that requires an even lower pH.
I've tested water before and after our calcium reactors, just to see if the NO3 is consumed in there, but didn't show any differences.
How to dissolve the CO2? A bubble a guess would just go through the sand up to the surface.
value xxxx EDIT (should have said the pH probe). They originally believed that the calcium carbonate substrate would disolve & maintain alk & cal, but I think that supplimenting is still necessary.
Last edited:
Yes I know about that. Visited the Rotterdam Zoo and saw the large experimental tank, the precursor of the Dymico filter. So they have been an inspiration for a long timeDyminco inject co2 straight into the substrate, computor controlled via the orp probe value. They originally believed that the calcium carbonate substrate would disolve & maintain alk & cal, but I think that supplimenting is still necessary.
But I think they recirculated the plenum water with quite large pumps. So in that case it wouldn't be a problem to break up the CO2 bubbles.
My sketch is too low flow for that. But maybe it's possible to use an upside down bowl in the plenum so the CO2 stays.
/ David
I see the Dymico system has evolved quite a bit over the years. They now have a blue box version for systems as small as 200 litres.Yes I know about that. Visited the Rotterdam Zoo and saw the large experimental tank, the precursor of the Dymico filter. So they have been an inspiration for a long time
But I think they recirculated the plenum water with quite large pumps. So in that case it wouldn't be a problem to break up the CO2 bubbles.
My sketch is too low flow for that. But maybe it's possible to use an upside down bowl in the plenum so the CO2 stays.
/ David
I found the idea intriguing when i first saw it & I've been curious about it ever since. But it is high tec being computor controlled & all, & at the end of the day i think its unnecessarily complicated considering its main purpose is basically just inorganic nutrient control.
Last edited:
Here's an idea I want to try more. I've tried it once but put a layer of Siporax in the bottom and it turned out Siporax release a lot of silicate in anaerobic conditions. So I had to stop it and havn't had the time or place to try it again. The idea is a box inside the sump, with inlet at the bottom. Water is pumped out from the top water layer. This way it's easier to know how much water goes through the sand bed, and it's easy to put pH and redox probes to see the numbers after the sand bed. You could also feed the sand bed with carbon source and regulate the dosage and flow with the pH and redox(maybe). Anyway, not easy to explain in text so I draw this in power point
I call it David's reverse deep sand bed, or just DRDSB
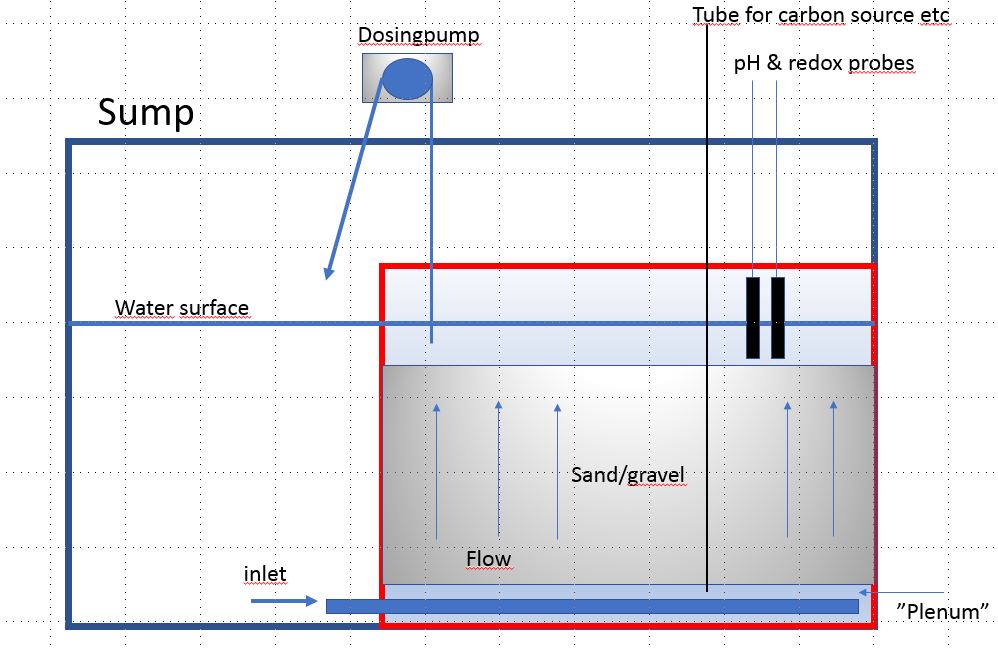
Refresh my thinking, if what I say is off alittle. First I think, you need to determine in relation to water movement how fast to move water, this can be determined by orp of course this done last when it's up and running . If going in my tank here's my add on's to this design. We know anaerobic starts, 1 cm at least from the surface. I would add a a pipe(s) in middle of anaerobic activity. Recirculate to bottom at plenum. To increase bacteria activity or from other end where water exits,pipe from there to plenum, or have water entering, go thru coil piping to remove oxygen before entering plenum. That way anaerobic starts quicker. The water exiting my be 1-3 gallons an hour. My 2 cents
Last edited:
Absolutely. I think the flow could be improved, both like you write by recirculate water to the plenum and also think about how to spread the flow as much as possible over the sand bed. My sketch is just an idea, I'm sure it can be improved a lot.Refresh my thinking, if what I say is off alittle. First I think, you need to determine in relation to water movement how fast to move water, this can be determined by orp of course this done last when it's up and running . If going in my tank here's my add on's to this design. We know anaerobic starts, 1 cm at least from the surface. I would add a a pipe(s) in middle of anaerobic activity. Recirculate to bottom at plenum. To increase bacteria activity or from other end where water exits, or have water entering go thru coil piping to remove oxygen before entering plenum. That way anaerobic starts quicker. The water exiting my be 1-3 gallons an hour. My 2 cents
Sorry @Subsea for hijacking your thread
/ David
Thanks @Subsea. Someone opened a can of worms.
Where detritus settles and recirculation , flow too. Key to work properly. MO
Forgot amount of detritus settling too
Forgot amount of detritus settling too
I'm not as dumb as I speak. For a Mexican/Jew. HeeheeAbsolutely. I think the flow could be improved, both like you write by recirculate water to the plenum and also think about how to spread the flow as much as possible over the sand bed. My sketch is just an idea, I'm sure it can be improved a lot.
Sorry @Subsea for hijacking your thread
/ David
- Joined
- Sep 7, 2014
- Messages
- 994
- Reaction score
- 655
So you would have a “carbon dosed” tank without having to carbon dose?My goal is to create the first (as I know) self playing denitrification system in aquaria (No need of external add of DOC). But it will take time.
How about co2 injection to maintain an anoxic zone for denitrification.
Help me understand why co2 injection. I thought co2 required photosynthesis for glucose production. What is going on in the dark?
Previously, when I used passive Jaubert Plenum with 6” dsb, the theory was to extend the oxygen gradient over a larger volume by using larger grain aroggonite at 2-4 mm, thus allowing more oxygen deeper. Since denitrification was the goal, using facultative bacteria. I never followed the anarobic side of the chemistry. Today, I have no need for denitrification as I add nitrogen in addittion to heavy feeding.
On my newest tank at 7 months old, I used a false bottom with 2” of aroggonite from ten year old system with a small flow into Plenum thus a reverse flow. Many worms grow in this substrate providing grazing for drawf angels and mandarin.
Last edited:
So you would have a “carbon dosed” tank without having to carbon dose?
I must inject my thoughts on dosing organic carbon. It grows bacteria, not coral.
Carbon dioxide dissolves in water to form carbonate & bicarbonate alkalinity which combines during photosynthesis to form glucose which is carbon for the reef.
Aroggonite begins to dissolve at a pH of 8.05. The pH between daylight and dark fluctuates from
8.15 - 7.85. I get passive buffering from aroggonite sandbed by doing nothing.
PSS. I just realized you designed a giant calcium reactor.
8.15 - 7.85. I get passive buffering from aroggonite sandbed by doing nothing.
PSS. I just realized you designed a giant calcium reactor.
I'm all up for trying that as well. Another thing is if the pH gets low enough to dissolve some Ca, Mg etc from the coral gravel, you got a calcium reactor. I have no idea if that's possible, maybe that requires an even lower pH.
I've tested water before and after our calcium reactors, just to see if the NO3 is consumed in there, but didn't show any differences.
How to dissolve the CO2? A bubble a guess would just go through the sand up to the surface.
The Dimico system uses this method to controll the oxygen level in the substrate so it is in an anoxic state (perfect for denitrifying bacteria), not anaerobic or oxic. An orp probe reading will give various readings depending on the level of oxygen in a given environment. The orp probe is connected to the Dimico computor as is the source of co2 injection.Help me understand why co2 injection.
I have a background of running an experimental waste water treatment plant (small - serving around 200 individuals - Stensunds wastewater aquaculture) between 1993 and 2000 and in construction of indoors recirculation fish farms with total nitrogen removal from the water.
Sincerely Lasse
The bugs do it all. A microbiologist friend said of the bugs, “Microbial Overlords”. I say, just ask the Martians in “War of the World”.
Many years ago, I worked at a Schreiber activated sludge plant. They stressed bacteria by turning air bubbles off, thus stressing bacteria with a resulting uptake of phosphate. As an operator, we controlled by dissolved oxygen which was the largest consumer of energy.
I understand what you said. I do not understand why more co2 causes less oxygen.The Dimico system uses this method to controll the oxygen level in the substrate so it is in an anoxic state (perfect for denitrifying bacteria), not anaerobic or oxic. An orp probe reading will give various readings depending on the level of oxygen in a given environment. The orp probe is connected to the Dimico computor as is the source of co2 injection.
Good question; I don't believe it does.I understand what you said. I do not understand why more co2 causes less oxygen.
The co2 injection is to controll pH so the system acts as a calcium reactor.
I got mixed up with the carbon dosing & co2 injection part, sorry.
To maintain optimal ORP values for denitrification the computer system modulates water flow through the filter, and also carbon injection of the reactor.
Similar threads
- Replies
- 2
- Views
- 102
- Price: SOLD
- Shipping NOT Available
- Replies
- 0
- Views
- 385

















