So I have never been too serious about testing my tank (old habits), however I decided I needed to get with the decade. I bought a trident and it fixes my semi color blindness issue.
so with that being said I unknowingly had a separated batch of IO that jumped my alk to 15+/Ca 600+/mag 1600+ and that’s why I started monitoring.
Over the course of the last month I have done 2 gal auto water changes and switched salt to tropic Marin reef pro which has resulted in me getting the parameters in satisfactory rang
my results are now
alk - 9.5 (my target)
Mag - 130 (target of 1325)
Ca - 539 (this one has me stumped)
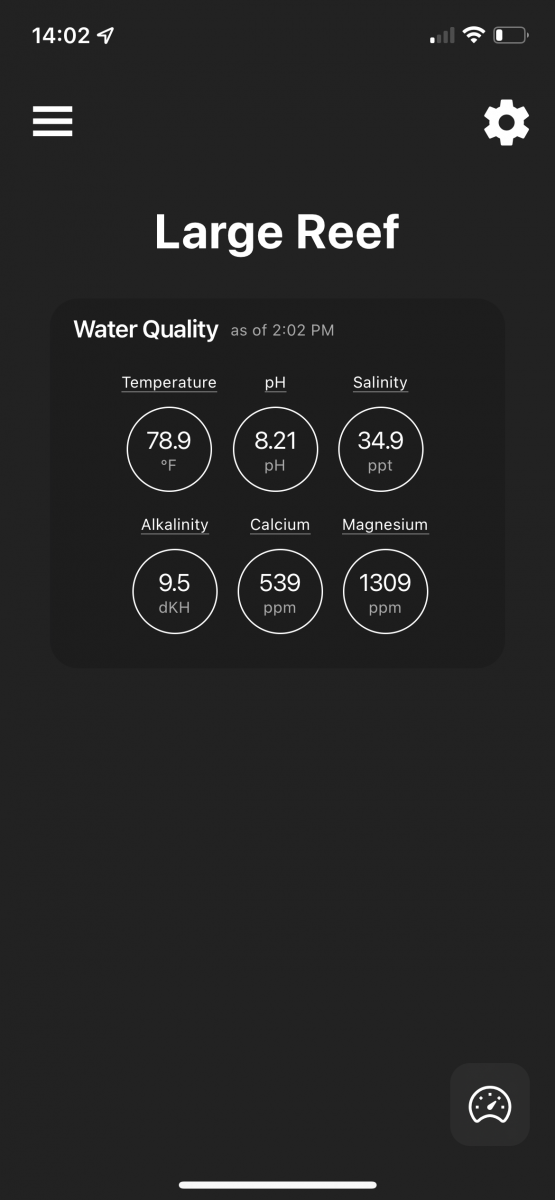
I have a heavily populated mixed reef and after getting my Alk down below 10 my sps are visibly growing every day with recordable change. This clearly tells me calcium is being used but it’s still so much higher than my target (425).
I am set up to dose B-ionic 2 part, but I keep holding back on starting the schedule until the calcium gets to near what I want. I know 1 part is Alk and the other is ca/mag together, and if you notice my results the mag is below target. Should I dose just mag for now until ca drops more?
Would love thoughts/opinions/feedback thanks!
so with that being said I unknowingly had a separated batch of IO that jumped my alk to 15+/Ca 600+/mag 1600+ and that’s why I started monitoring.
Over the course of the last month I have done 2 gal auto water changes and switched salt to tropic Marin reef pro which has resulted in me getting the parameters in satisfactory rang
my results are now
alk - 9.5 (my target)
Mag - 130 (target of 1325)
Ca - 539 (this one has me stumped)
I have a heavily populated mixed reef and after getting my Alk down below 10 my sps are visibly growing every day with recordable change. This clearly tells me calcium is being used but it’s still so much higher than my target (425).
I am set up to dose B-ionic 2 part, but I keep holding back on starting the schedule until the calcium gets to near what I want. I know 1 part is Alk and the other is ca/mag together, and if you notice my results the mag is below target. Should I dose just mag for now until ca drops more?
Would love thoughts/opinions/feedback thanks!


















