I'm using an online calculator for CO2 and the results are considerable less than the Hach titration method. The CO2 meter I bought (for air) seems to be useless.Any updates on this Dana? I'm getting curious about the same and wonder if you came up with anything.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Anyone monitoring aquarium CO2 (carbonic acid)?
- Thread starter Dana Riddle
- Start date
- Tagged users None
Most likely your indoor air quality (CO2) is good. Almost all newer houses have some sort of air re-circulation method to deal with CO2 build up. It is the older houses that usually have problems. You can buy decent air quality devices that will give you a decently accurate measure as to your indoor CO2. I use one by Air Things and it works well.This does nothing to improve ph. on my system / please advise
Dissolved oxygen tests are expensive and the cheap ones don't give a granular number to work with. Also you need to be testing regularly through out the day to get a good feel for your levels.I do want to add why dint we ever measure dissolved oxygen in our reef tanks .
Useless how? Not accurate enough?I'm using an online calculator for CO2 and the results are considerable less than the Hach titration method. The CO2 meter I bought (for air) seems to be useless.
How do you have it hooked up? IE, are you bubbling tank water into a closed loop with the air sensor or similar? That's what I was considering, but if it doesn't work then no point.I'm using an online calculator for CO2 and the results are considerable less than the Hach titration method. The CO2 meter I bought (for air) seems to be useless.
I am just monitoring air in the lab. That's an interesting idea you have. I think I have time to get into the lab tomorrow and I'll test your thought.How do you have it hooked up? IE, are you bubbling tank water into a closed loop with the air sensor or similar? That's what I was considering, but if it doesn't work then no point.
This is the calculator I'm using.I am thinking about Your CO2 online calculator. If it is for fresh water it shows wrong because the pH for a given kH is lower in marine water.
Marine CO2 Level Calculator
Aquarium calculator; Estimate the carbon dioxide (CO2) concentration given a few relevant parameters.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,142
- Reaction score
- 63,494
The simplistic comment that it cannot possibly work in seawater at CO2 levels below some point, because seawater at pH 8.3 and up definitely has free CO2, yet this method would claim it has none.
The page you posted says that in seawater (top of page two,) the high salt content causes "negative errors". The method assumes no carbonic acid remains at pH 8.3, and yet, seawater at pH 8.3 has ALL of its carbonic acid remaining.
The simplistic comment that it cannot possibly work in seawater at CO2 levels below some point, because seawater at pH 8.3 and up definitely has free CO2, yet this method would claim it has none.
The page you posted says that in seawater (top of page two,) the high salt content causes "negative errors". The method assumes no carbonic acid remains at pH 8.3, and yet, seawater at pH 8.3 has ALL of its carbonic acid remaining.
Thanks Randy. Using this titration method, I get a result of perhaps 40 mg/L. Using the online calculator, I get less than 1 mg/L. Hach's motto is "Be Right." Makes me wonder.The simplistic comment that it cannot possibly work in seawater at CO2 levels below some point, because seawater at pH 8.3 and up definitely has free CO2, yet this method would claim it has none.
The page you posted says that in seawater (top of page two,) the high salt content causes "negative errors". The method assumes no carbonic acid remains at pH 8.3, and yet, seawater at pH 8.3 has ALL of its carbonic acid remaining.
- Joined
- May 22, 2016
- Messages
- 6,526
- Reaction score
- 10,060
That's nice. If only it had pCO2 as well to compare the measurement of air CO2 with what's in the water.This is the calculator I'm using.
Marine CO2 Level Calculator
Aquarium calculator; Estimate the carbon dioxide (CO2) concentration given a few relevant parameters.www.hamzasreef.com
When I looked at it, I found that my water CO2 - from the pH + titration was reflective of the large variations in the indoor air CO2 (room might have a couple dozen people in it), but with a ~2hr delay.
Thank you! I was wondering if that calculator accounted for atmospheric CO2. The CO2 in the lab's air is often greater than 1200 ppm.That's nice. If only it had pCO2 as well to compare the measurement of air CO2 with what's in the water.
When I looked at it, I found that my water CO2 - from the pH + titration was reflective of the large variations in the indoor air CO2 (room might have a couple dozen people in it), but with a ~2hr delay.
Did you get a chance to test this? I’m thinking of trying something similar and figured I’d see if you had any luck first.I am just monitoring air in the lab. That's an interesting idea you have. I think I have time to get into the lab tomorrow and I'll test your thought.
I did - and still am. I'll get into the lab this morning and post results.Did you get a chance to test this? I’m thinking of trying something similar and figured I’d see if you had any luck first.
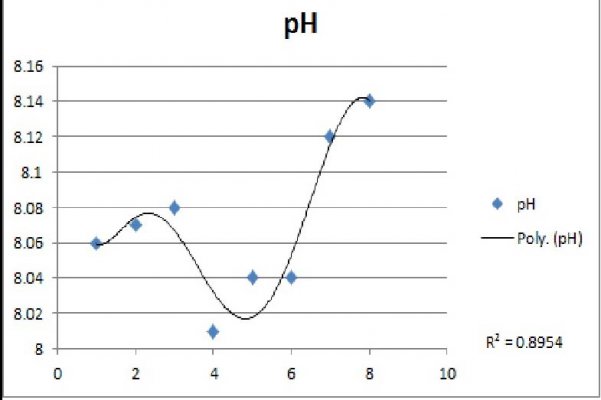
A 1-liter volumetric cylinder was filled with artificial seawater from my reef aquarium and aerated by a small air pump fitted with a coarse bubble air stone. A Hach pH meter monitored pH. The pH sensor was calibrated with 7.01 and 10.01 buffers. Atmospheric CO2 was measured with a air quality detector. Temperature of the water sample remained more or less constant at ~23C. Over the course of two days, the pH rose from a low of 8.06 to a high of 8.15. Atmospheric CO2 was not constant, with reported low and high of 691 ppm and 1356 ppm, respectively. I observed that CO2 concentrations would rise merely by my presence, hence there is no correlation of water pH and atmospheric CO2 that I can see. In addition, I have 20-gallons of green water under culture in the lab. Perhaps a more detailed examination would yield results that make sense.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,142
- Reaction score
- 63,494
A 1-liter volumetric cylinder was filled with artificial seawater from my reef aquarium and aerated by a small air pump fitted with a coarse bubble air stone. A Hach pH meter monitored pH. The pH sensor was calibrated with 7.01 and 10.01 buffers. Atmospheric CO2 was measured with a air quality detector. Temperature of the water sample remained more or less constant at ~23C. Over the course of two days, the pH rose from a low of 8.06 to a high of 8.15. Atmospheric CO2 was not constant, with reported low and high of 691 ppm and 1356 ppm, respectively. I observed that CO2 concentrations would rise merely by my presence, hence there is no correlation of water pH and atmospheric CO2 that I can see. In addition, I have 20-gallons of green water under culture in the lab. Perhaps a more detailed examination would yield results that make sense.
I'm confused. Why do you say there is no correlation?
Was the alkalinity constant? Supplemented at all? Was photosynthesis happening?
Even in the absence of any internal changes in CO2 (say, photosynthesis or respiration or supplement additions) or alkalinity changes (calcification, supplementation), there will be a lag between atmospheric CO2 changes and aquarium pH changes because it takes time to equilibrate an aquarium. CO2 is fairly hard to equilibrate (which is the reason there is a day to night change in pH in most aquaria).
I'm confused. Why do you say there is no correlation?
Was the alkalinity constant? Supplemented at all? Was photosynthesis happening?
Even in the absence of any internal changes in CO2 (say, photosynthesis or respiration or supplement additions) or alkalinity changes (calcification, supplementation), there will be a lag between atmospheric CO2 changes and aquarium pH changes because it takes time to equilibrate an aquarium. CO2 is fairly hard to equilibrate (which is the reason there is a day to night change in pH in most aquaria).
No, I don't think it's you who is confused Randy. LOL. It seems apparent (at least to me) that just aerating a sample and watching pH shifts is not enough. pH initially rose, then fell, and finally rose. pH was highest when air CO2 was highest - this first thing in the morning after lights being off all night.I'm confused. Why do you say there is no correlation?
Was the alkalinity constant? Supplemented at all? Was photosynthesis happening?
Even in the absence of any internal changes in CO2 (say, photosynthesis or respiration or supplement additions) or alkalinity changes (calcification, supplementation), there will be a lag between atmospheric CO2 changes and aquarium pH changes because it takes time to equilibrate an aquarium. CO2 is fairly hard to equilibrate (which is the reason there is a day to night change in pH in most aquaria).
Attachments
Dana, I didn't explain very well, and I don't think I conveyed what I meant to. Please see these two diagrams. The version with the CO2 meter assumes that a correlation between air concentration of CO2 and salt water concentration of CO2 can be determined. The version with the pH meter assumes that while this correlation is not known, that it is the same in fresh and salt mater. I do not know if this is the case. The air pump and aquarium side airstone in both of these cases could be a skimmer with the lid set up as a closed loop back to the intake as we often do with CO2 scrubbers.
With pH probe. This is not unlike how a drop checker works in a planted freshwater tank, although in that case the pH probe is typically replaced with a color changing pH reagent. It is also normally done without air being forced through the water, and so exhibits a long delay.
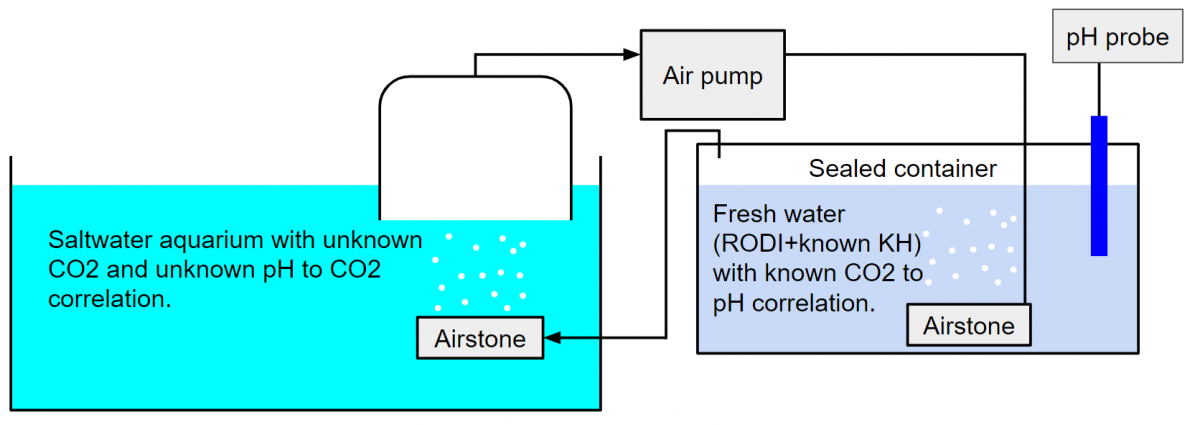
With CO2 sensor:
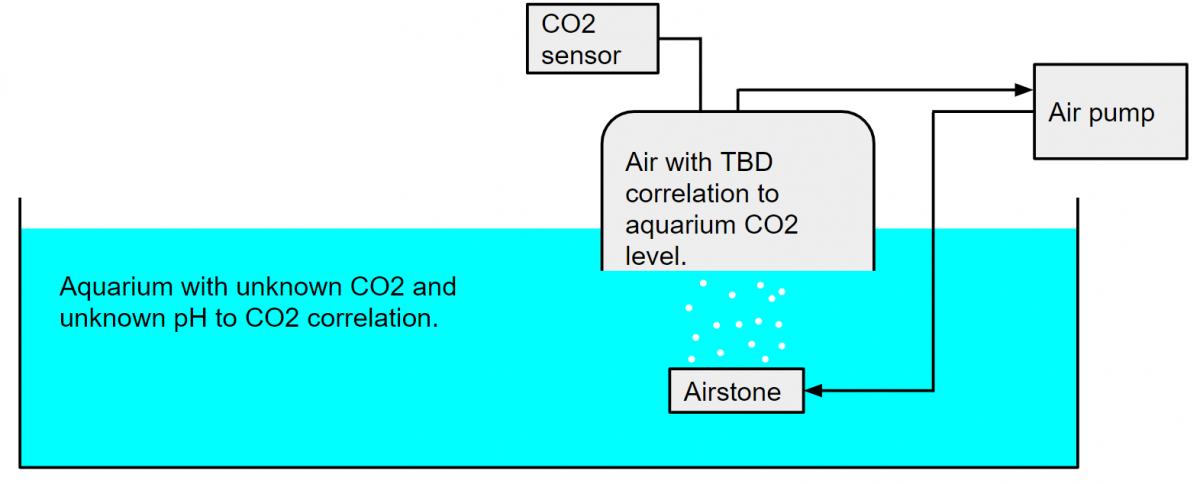
With pH probe. This is not unlike how a drop checker works in a planted freshwater tank, although in that case the pH probe is typically replaced with a color changing pH reagent. It is also normally done without air being forced through the water, and so exhibits a long delay.
With CO2 sensor:
I should add that the fluid in the sealed container (pH probe version) does not last forever. With a drop checker using bromothymol blue I've observed the fluid getting stuck either yellow (low pH) or blue (high pH). I don't typically see much growth, but I have seen the water level change. My first guess is that this is aquarium water crossing to or from the sealed container. Drop checkers are normally only a few mL, so a small increase or decrease in volume could make an appreciable difference in hardness, which of course changes the CO2 to pH correlation. Changing the fluid out once a month or two resolved this issue for me. With forced air this may need to be sooner, but with a larger total volume it may be a non issue. My CO2 scrubber has multiple chambers and while the first chamber regularly collects condensation, the second and third ones never do, so a water trap upstream may solve the problem as well. I always used 4 dKH water to put the yellow/green color shift at a 6.8 pH around 30 PPM CO2 (I was adding it), but you can tune this by using softer water.
Similar threads
- Replies
- 1
- Views
- 170
- Replies
- 20
- Views
- 901
- Replies
- 8
- Views
- 258
- Replies
- 16
- Views
- 370