- Joined
- Sep 11, 2015
- Messages
- 538
- Reaction score
- 260
Got my ATI ICP test back, and shows lows iodine, which I have resolved by dosing some over a couple days. I am still pretty stumped on the Tin:
RODI water:
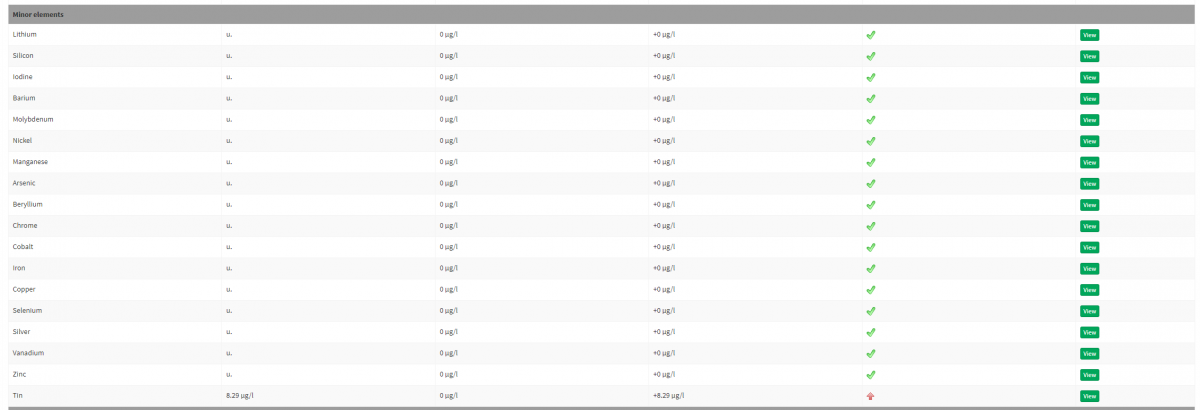
From my RODI water, shows 8.29 UG/L. I switched out everything on my RODI unit about 2-3 months prior to this test (except the membrane). I looked for any hinges, screws, rust, etc, and nothing seems to be the culprit for the 8.29 number. My RO water container which I fill up that my tank pulls from is a Innovative Marine AUQA Gadget HydroFill Ti Auto Top Off ATO Reservoir - 15 Gallon, which could MAYBE be leaching Tin from glass (Heard float glass can leach tin) -- but I had this unit for about a year so far. I checked the Apex pump in the reservoir, and found no rust on it either.
My Tank results:
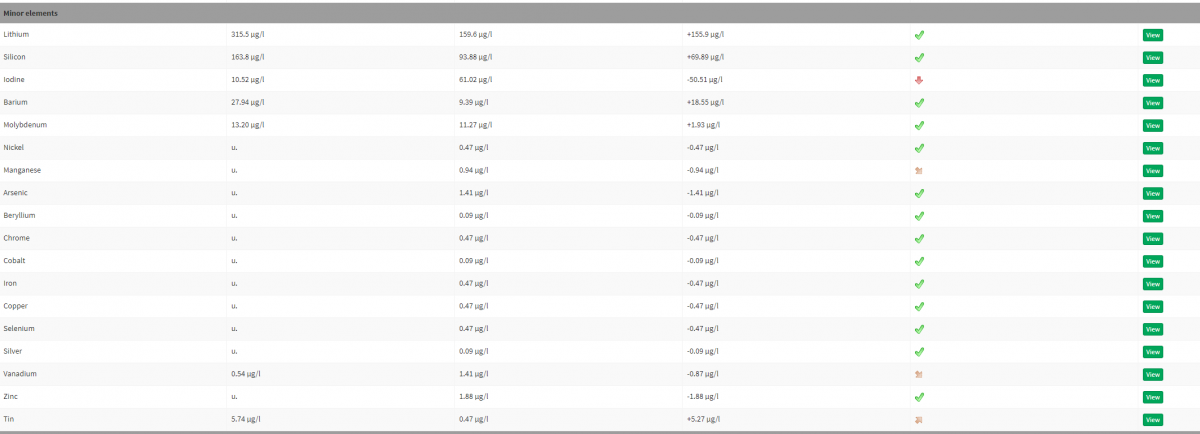
My tank results show Tin, but at a lower level at 5.74 ug/l. The only thing I can find in my sump, is a screw that was rusted that was holding up my UV light. I replaced the screw with SS screws, but i doubt this was the cause for the Tin in the tank.

My assumption is, when the ATO unit is putting RODI water back into the tank, it's slowly adding Tin into it (since it was 8.74 in the test). Am I missing something? Where could the Tin be coming from? Should I replace my Membrane in the RODI unit? It's been about 2 years, but still showing 0 TDS. Currently my pressure on the unit is 60 PSI. This was the minimum required, so I ordered a booster to get it upt to 80 psi. This might help with better filtration in removing Tin?
Any help / thoughts would be great.
@Randy Holmes-Farley
RODI water:
From my RODI water, shows 8.29 UG/L. I switched out everything on my RODI unit about 2-3 months prior to this test (except the membrane). I looked for any hinges, screws, rust, etc, and nothing seems to be the culprit for the 8.29 number. My RO water container which I fill up that my tank pulls from is a Innovative Marine AUQA Gadget HydroFill Ti Auto Top Off ATO Reservoir - 15 Gallon, which could MAYBE be leaching Tin from glass (Heard float glass can leach tin) -- but I had this unit for about a year so far. I checked the Apex pump in the reservoir, and found no rust on it either.
My Tank results:
My tank results show Tin, but at a lower level at 5.74 ug/l. The only thing I can find in my sump, is a screw that was rusted that was holding up my UV light. I replaced the screw with SS screws, but i doubt this was the cause for the Tin in the tank.
My assumption is, when the ATO unit is putting RODI water back into the tank, it's slowly adding Tin into it (since it was 8.74 in the test). Am I missing something? Where could the Tin be coming from? Should I replace my Membrane in the RODI unit? It's been about 2 years, but still showing 0 TDS. Currently my pressure on the unit is 60 PSI. This was the minimum required, so I ordered a booster to get it upt to 80 psi. This might help with better filtration in removing Tin?
Any help / thoughts would be great.
@Randy Holmes-Farley





















