Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,256
- Reaction score
- 63,602
I this really a study we need to do? Do not we know quite much about the mechanism of calcification so we already have the answer? Maybe I am wrong, I am not sure, but I would guess that a study like this would show that the alkalinity consumption would still be lower in the night, BUT the difference would be a little lower than in a natural system with pH difference. I think in this way: There are 2 forces to consumption alk. First the spontaneous which is very much driven my a higher pH, and there is the biological. The biological, thus in the coral, is also favored by higher pH in the environment water as far as I have understood, as it is more easy for the coral to "through out" the H+ from the obtained HCO3, to get CO3, and thus create CaCO3. But, as the coral also have a zooxanthella, and we know by studies that the photosynthesis in zooxanthelles have positive effect on formation of CacO3, the light itself also will improve the CaCO3 formation even if the pH in the water in the tank is of a lower pH, as the local pH in the coral, in the CaCO3-fixations local process, hav a higher pH due to the zoox local consumption of co2. Therefore I do think that the light will have an impact of alk-consumption even if the pH in environmental water is lower. So, this experiment you talk about (which of course is interesting, all exp are), I think will give the results that alk consumption is still some lower at night, BUT the difference comparing to a natural system, is slight lower, as even if the coral have internal CaCO3 formations process it is probably more easy for it if also the environment water have a higher pH.
And: I have seen very often the scenario that when we increase light, we got a remarkable increase of alkalinity consumption(if you have a lot of sps), but NOT that corresponding increase in pH. That gives some fuel to my thoughts above. The light itself due to the zoox have a very high potential to favorite CaCO3 in the coral, and it is not so dependent of the surrounding pH. That is maybe a very good survival strategy of the coral, and one of the reasons of the zoox symbiotic partnership. Of course there are limits, where ocean acidification get ot more difficult for the coral. It can not compensate a low pH in the water over a certain point of course, thus: if we in your experiment idea settle the pH to a too low level, I think we reach a point where there is no difference in alk consumption during day and night...but then we have reached the point where the coral due to too low pH cannot calcify despite the light.
/Jonas
I think we agree that the abiotic precipitation will increase when pH is highest. What portion of the total alk consumption does that comprise in a typical reef tank? 5%? 75%? It might vary hugely.
It is certainly established in the scientific literature that photosynthesis "helps" calcification. The question is why and whether higher external pH may be a driver.
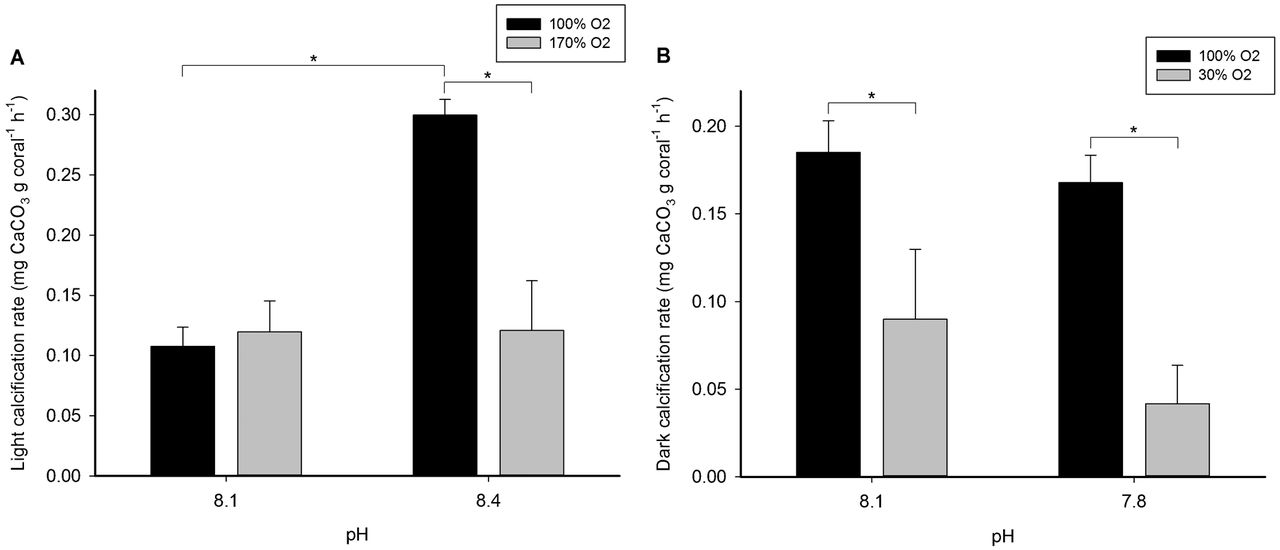
Here's a recent paper that shows the dark calcification rate is actually higher than the light calcification rate at fixed pH 8.1. for an acropora (see Figure 1). So might the effects that Jim and others see showing more alk demand during the day not actually reflect increased calcification during the day, but some other process, potentially including abiotic precipitation? Or maybe O2 is the driver?
http://bio.biologists.org/content/3/6/489
Fig. 1.Calcification rates of Acropora millepora.
(A) Light calcification rate of A. millepora at a pH of 8.1 and 8.4, and 100 and 170% oxygen saturation. (B) Dark calcification rate of A. millepora at a pH of 8.1 and 7.8, and 100 and 30% oxygen saturation. Values are means + S.E. (n = 4). Asterisks indicate significant differences (P<0.050). The light and dark experiments were carried out with an interlude of several weeks.