Hello R2R,
I come with a strange situation that me nor my local reef friends can figure out. My new tank has been up and running for 3 months or so - at this point it's lightly stocked on both the Fish and Coral front.
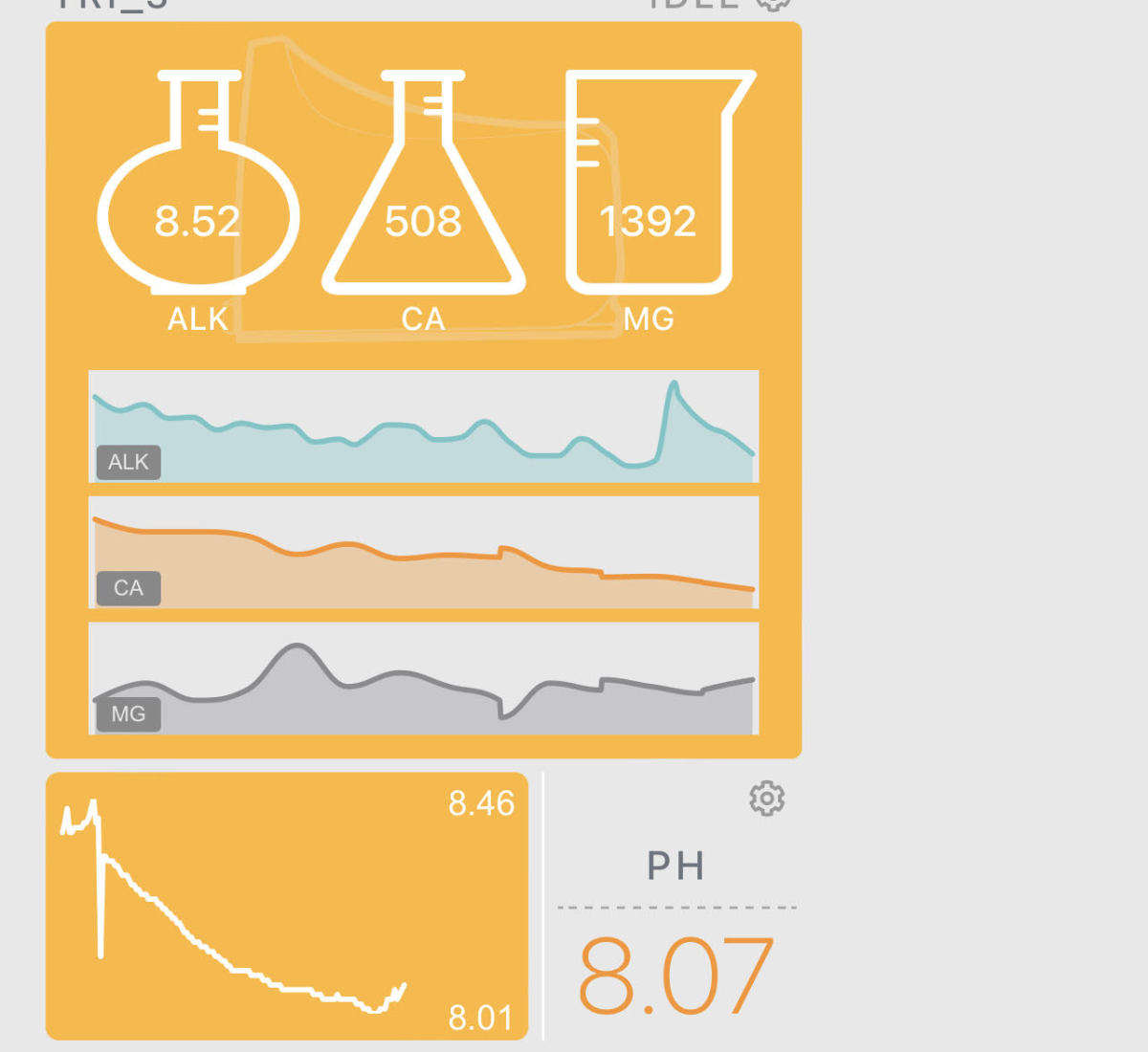
My ALK keeps on falling and falling despite adding around 300ml of concentrated aquaforest. I just recently switched to the concentrated formula after I realized that my tank was rapidly eating the alk.
I'm attaching a screen shot of my levels - last night I manually dosed in alk to get the level up to ~9.6 or so, and my most recent test (not even 24 hours later) read out at 8.5. (hannah confirmed)
My Calcium is 500 and above what I'd normally be running it at thanks to swapping over to the pro formula of aquaforest - I'm slowly letting that come down. MG is hovering around 1400 which is my target - daily swings of around 200 or so is expected.
I'm including a screen shot of my apex below so you can see for yourselves. It's a real head scratcher - my coral are all thriving and I'm even seeing new growth out of my acros - but long term this can't continue happening.
I'm going to do a triton test as soon as these levels settle around my goals but until that happens I'm holding off.
This is an assumption on my part but the only thing that makes a little sense to me is my substrate / rocks are absorbing it in some way... I have florida crushed coral on top of the ocean direct sand with Caribsea rocks... I don't logically believe that but I can't figure it out!
PH hovers between 8 and 8.5 which implies that CO2 isn't an issue.
Let me know if there's anything else missing <3
I come with a strange situation that me nor my local reef friends can figure out. My new tank has been up and running for 3 months or so - at this point it's lightly stocked on both the Fish and Coral front.
My ALK keeps on falling and falling despite adding around 300ml of concentrated aquaforest. I just recently switched to the concentrated formula after I realized that my tank was rapidly eating the alk.
I'm attaching a screen shot of my levels - last night I manually dosed in alk to get the level up to ~9.6 or so, and my most recent test (not even 24 hours later) read out at 8.5. (hannah confirmed)
My Calcium is 500 and above what I'd normally be running it at thanks to swapping over to the pro formula of aquaforest - I'm slowly letting that come down. MG is hovering around 1400 which is my target - daily swings of around 200 or so is expected.
I'm including a screen shot of my apex below so you can see for yourselves. It's a real head scratcher - my coral are all thriving and I'm even seeing new growth out of my acros - but long term this can't continue happening.
I'm going to do a triton test as soon as these levels settle around my goals but until that happens I'm holding off.
This is an assumption on my part but the only thing that makes a little sense to me is my substrate / rocks are absorbing it in some way... I have florida crushed coral on top of the ocean direct sand with Caribsea rocks... I don't logically believe that but I can't figure it out!
PH hovers between 8 and 8.5 which implies that CO2 isn't an issue.
Let me know if there's anything else missing <3
















