- Joined
- May 22, 2016
- Messages
- 6,545
- Reaction score
- 10,099
I saw this article, and thought it was interesting but didn't have near enough info to make it clear what was going on
https://phys.org/news/2023-04-big-sponge-co2-tech-oceans.html
This write-up gives a bit more detail on the basic idea for the CO2-stripping with electrochemical cells (but isn't about the specific project in the first article)
https://www.chemistryworld.com/news...xide-extract-it-from-seawater/4017113.article
And the underlying paper for the chemistryworld article works out the mechanics in plenty of detail....
Asymmetric chloride-mediated electrochemical process for CO2 removal from oceanwater (Kim et al)
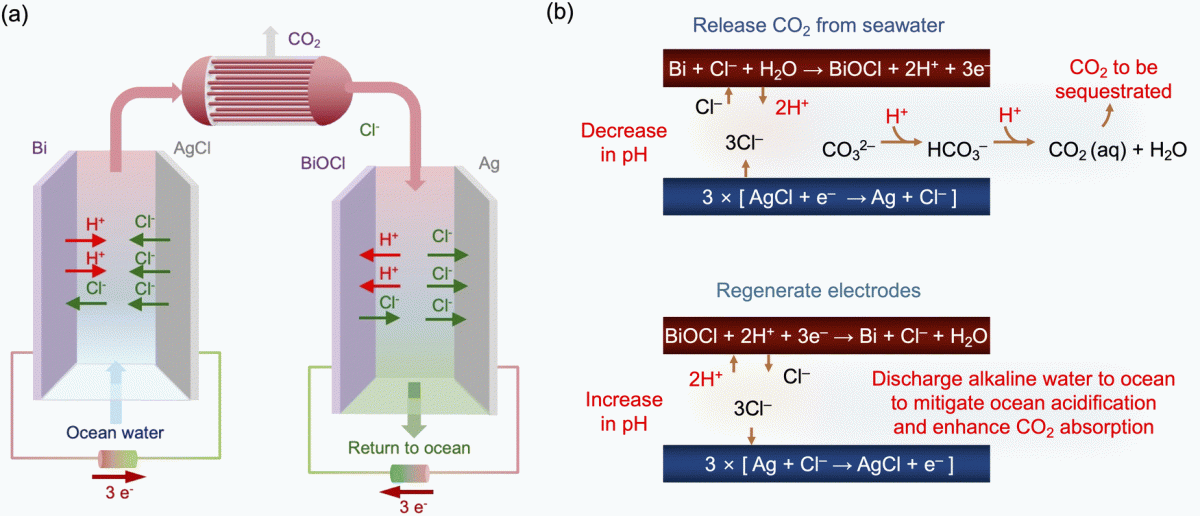
(process overview)
The basics are (I think) that they use electricity in one cell to acidify ocean water to low enough pH that all the carbonate/bicarbonate becomes CO2 in solution, then they strip out the CO2 gas (in the paper, they strip CO2 out by vacuum).
Then they take this CO2-free acidified seawater and put it to the other electrochemical cell where the charges go the opposite way and they raise the pH back. Then they have CO2-free good "alkaline" seawater that they dump back in the ocean. But it doesn't look to be "alkaline" in the sense of having much alkalinity the way we use that term in the hobby - there's no carbonate/bicarbonate going back into the seawater it seems?
The floating plant that's being operated in the first link (phys.org) article is doing this process on a boat that moves around so it isn't stuck doing this to too much water in one spot.
The detailed Kim paper doesn't suggest what to do with the CO2 that you strip out by vacuum, but the boat is dumping it back in - in the form of solid CaCO3. So they are either adding a whole bunch of Calcium on the boat, or they are stripping a lot of Ca from seawater to turn all the CO2/carbonate/bicarbonate from the treated seawater into CaCO3?
It's cool that it's possible (and crazy that this might be way cheaper than other CO2 capture) but I'm not seeing how the chemistry of the treated water they are putting back in the ocean looks much like seawater?
https://phys.org/news/2023-04-big-sponge-co2-tech-oceans.html
This write-up gives a bit more detail on the basic idea for the CO2-stripping with electrochemical cells (but isn't about the specific project in the first article)
https://www.chemistryworld.com/news...xide-extract-it-from-seawater/4017113.article
And the underlying paper for the chemistryworld article works out the mechanics in plenty of detail....
Asymmetric chloride-mediated electrochemical process for CO2 removal from oceanwater (Kim et al)
(process overview)
The basics are (I think) that they use electricity in one cell to acidify ocean water to low enough pH that all the carbonate/bicarbonate becomes CO2 in solution, then they strip out the CO2 gas (in the paper, they strip CO2 out by vacuum).
Then they take this CO2-free acidified seawater and put it to the other electrochemical cell where the charges go the opposite way and they raise the pH back. Then they have CO2-free good "alkaline" seawater that they dump back in the ocean. But it doesn't look to be "alkaline" in the sense of having much alkalinity the way we use that term in the hobby - there's no carbonate/bicarbonate going back into the seawater it seems?
The floating plant that's being operated in the first link (phys.org) article is doing this process on a boat that moves around so it isn't stuck doing this to too much water in one spot.
The detailed Kim paper doesn't suggest what to do with the CO2 that you strip out by vacuum, but the boat is dumping it back in - in the form of solid CaCO3. So they are either adding a whole bunch of Calcium on the boat, or they are stripping a lot of Ca from seawater to turn all the CO2/carbonate/bicarbonate from the treated seawater into CaCO3?
It's cool that it's possible (and crazy that this might be way cheaper than other CO2 capture) but I'm not seeing how the chemistry of the treated water they are putting back in the ocean looks much like seawater?
















