- Joined
- Dec 28, 2016
- Messages
- 22,742
- Reaction score
- 21,908
Hypothesis: There will be no difference between Free ammonia levels as measured by a seachem alert badge in the presence of Prime (IN FRESH RODI WATER).
Background: Numerous experiments have been done which seem to suggest Prime does not lower ammonia as measured by various tests in saltwater. There has been a question about whether it might do so in Freshwater This study is designed to test this.
Method / Materials. 2 Seachem alerts, prepared. 2 quarts of RODI (pH 7.2, Ammonia 0, Temp Room (72)). Add various amounts of ammonia prime, and adjust pH (see below)
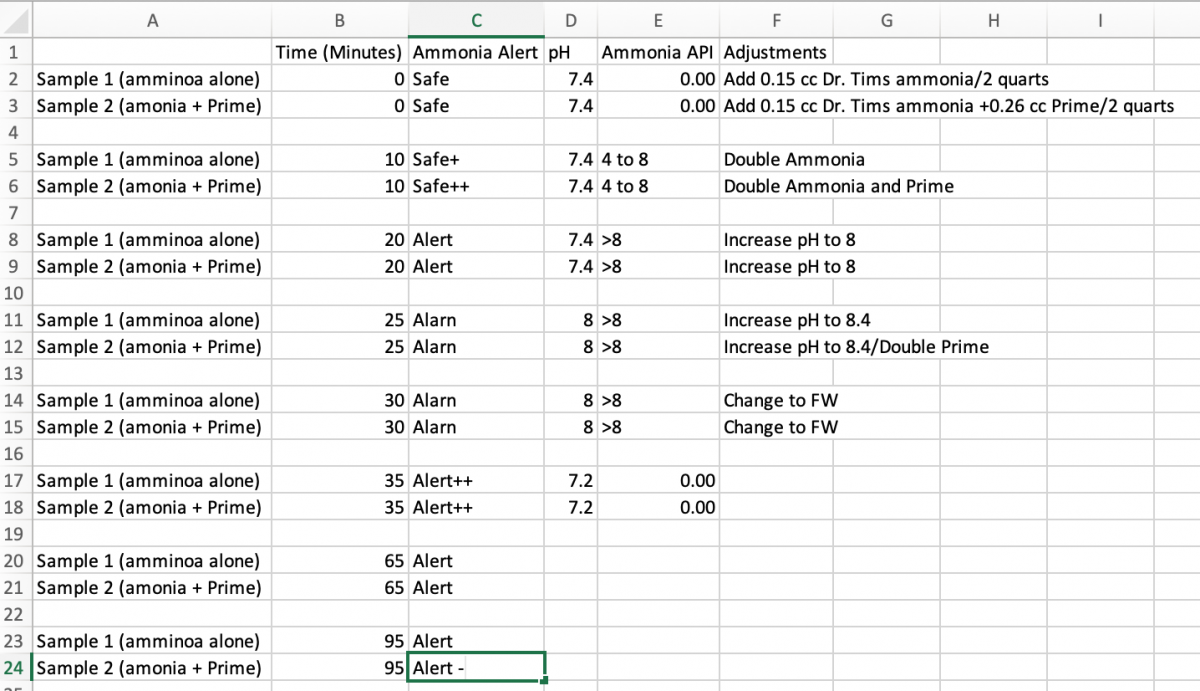
Results - see Excel Spreadsheet below
Conclusion:
1. Prime did not affect the seachem alert in fresh water over the time frames studied (I have heard it can take 2 hours for the full effect - but other places says immediately).
2. The Seachem alert performed well - and as expected with addition of bicarbonate to increase pH free ammonia rapidly increased.
3. The disk in the Prime sample seems to be recovering faster - after being added bas to fresh water. Whether this holds up or not - will see - when one or both gets to 'safe'.
4. A couple problems -
a. could have let the water sit with Prime longer
b. the ammonia level might have been 'too high'.
c. ammonia is not usually added to a disk all at once - it builds up - and its possible the immediate high concentration caused the film to change (however the water had prime added first - was mixed - then ammonia was added after)
d. Could the results have been different if I had started with pH 8.0 water added prime to one container - and then slowly - each hour added .25 or .5 ppm ammonia to each? I think this might also be an interesting experiment.
e. The experiment is not designed to test whether Prime works as its labeled or not.
Side Note - Will do the Multi-test tomorrow (the one Seachem recommends). Trying out that test today - I am thinking the test might be expired - though I don't see an obvious date (my RODI - tested at 2 ppm ammonia - and the reference tested higher than it should have as well).
Background: Numerous experiments have been done which seem to suggest Prime does not lower ammonia as measured by various tests in saltwater. There has been a question about whether it might do so in Freshwater This study is designed to test this.
Method / Materials. 2 Seachem alerts, prepared. 2 quarts of RODI (pH 7.2, Ammonia 0, Temp Room (72)). Add various amounts of ammonia prime, and adjust pH (see below)
Results - see Excel Spreadsheet below
Conclusion:
1. Prime did not affect the seachem alert in fresh water over the time frames studied (I have heard it can take 2 hours for the full effect - but other places says immediately).
2. The Seachem alert performed well - and as expected with addition of bicarbonate to increase pH free ammonia rapidly increased.
3. The disk in the Prime sample seems to be recovering faster - after being added bas to fresh water. Whether this holds up or not - will see - when one or both gets to 'safe'.
4. A couple problems -
a. could have let the water sit with Prime longer
b. the ammonia level might have been 'too high'.
c. ammonia is not usually added to a disk all at once - it builds up - and its possible the immediate high concentration caused the film to change (however the water had prime added first - was mixed - then ammonia was added after)
d. Could the results have been different if I had started with pH 8.0 water added prime to one container - and then slowly - each hour added .25 or .5 ppm ammonia to each? I think this might also be an interesting experiment.
e. The experiment is not designed to test whether Prime works as its labeled or not.
Side Note - Will do the Multi-test tomorrow (the one Seachem recommends). Trying out that test today - I am thinking the test might be expired - though I don't see an obvious date (my RODI - tested at 2 ppm ammonia - and the reference tested higher than it should have as well).
Last edited:


















