First post here on R2R! … I am new to the hobby but have been reading/studying it for years. I finally have space at home to begin. I will be following up in the future with build threads for my salt water mixing station, QT tanks (invert and fish), and display tank that I’m still building. I’m excited to finally post after 100s of hours of only reading.
I am having problems fishless cycling my coral quarantine tank - and it seems that I am possibly in a stalled cycle state. I have tried various substrates over the past 12 weeks and have monitored tank parameters almost daily. I have narrowed several things down and now suspect that Quaternary Ammonia Compounds (QACs) from fabric softening dryer sheets (standard Bounce Outdoor Fresh scented) have been transported into the tank through lint settling in the air. I want to get a read from the expert community if I’m on the right track here and I also want to understand the chemical mechanisms that could be at work.
Here are the details:

Figure 1: The Coral QT tank in Laundry Room.
I then added a couple of drops of QuicCycle to simulate a bioload to see if the tank would consume the ammonia. TA immediately spiked this time at 0.6 ppm and stayed there for over a week. Then it began going up! Wait! TA going up with no livestock? Something was wrong here. Over the next few days the TA reached 0.8 ppm and was there for several days. I continued to add supplemental bacteria and it did then go down over the next several days and this time the TA leveled off at 0.3 ppm - well above zero. At this point it felt like once again I had a stalled tank. I still had ammonia. I had helped bring it down with additional bacteria dosing but It was (and is still) stuck at TA of 0.3 ppm.
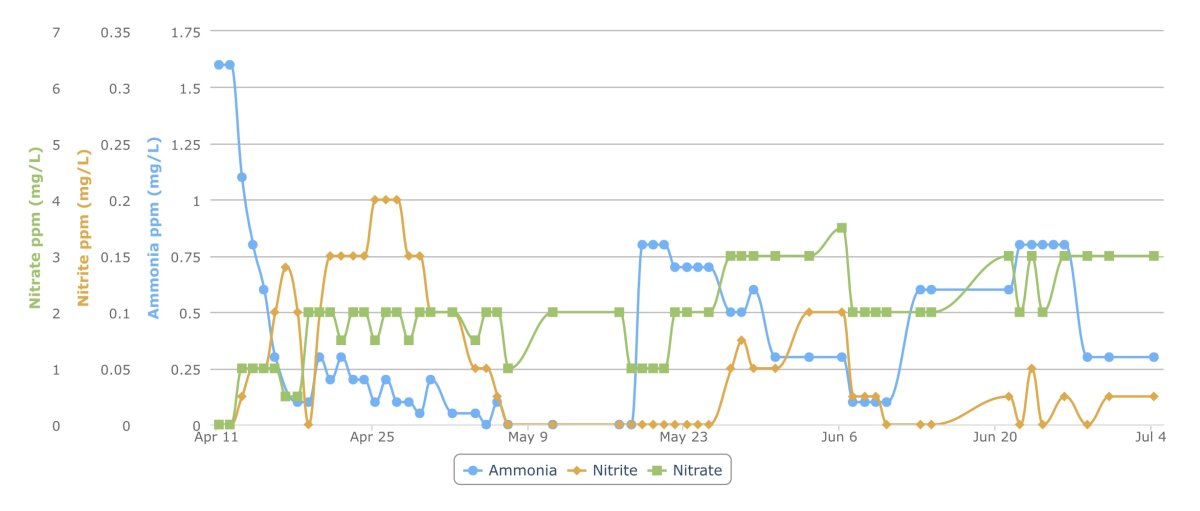
Figure 2: Cycling Data: Ammonia, Nitrite, and Nitrate over 12 weeks. Initial cycle and two subsequent stalled tests shown. There are a couple noisy/erroneous readings that are apparent in the data). Times of bacteria dosing not shown.
I then turned my attention to possible ammonia sources and thought that it could be introduced through the air. I first looked through the tank for any large bugs or any other organics that might have fallen into the tank but I found none. Recall, that this tank is in a laundry room and I next focused on this and especially on the chemicals in the room. The laundry detergents in use (sometimes containing bleach) are kept far away from the tank - but is it possible that bleach could transport through the air? This seemed unlikely but I wanted to rule it out. I also considered the fabric softener dryer sheets in use as there is a lot of dust (i.e. lint) that forms on horizontal surfaces in the room. (Note: There are a couple existing discussions on R2R about tanks in laundry rooms but there is nothing conclusive in those threads on the associated risks.)
I purchased the Total Chlorine ULR Hanna checker and got 5 ppb TC in the tank. This was higher than my target of keeping it below 2 ppb as I found some articles suggest this level for marine environments. Recall, I get 1 ppb TC out of my 7-stage RODI so the in-tank readings showed additional TC over the source RODI water. This 5 ppb TC reading was concerning since it was clearly above the suggested safe levels. It was (and still is) not clear what impacts this has on the tank, but my focus moved quickly to fabric softeners since what I found was even more concerning.
I mixed up a precise batch of saltwater and confirmed zero TA in it. I then pulled a small amount of lint from the lint trap in the dryer, soaked it in 100 ml of this saltwater for a few minutes and then tested for ammonia. I got high levels of TA at nearly 2 ppm (this test not shown in figures). I then went around the room and collected small amounts of dust (with a wet finger) from the tops of picture frames on the walls and transferred this dust/lint into 100 ml of salt water … this too tested high for TA at 0.8 ppm (shown in Figure 3). This testing was to discern the presence or lack thereof of ammonia in the lint/dust and it showed that it was clearly present and at levels that surprised me relative to the small amounts of lint that I collected. I did not control for the precise amount of lint/dust dissolved into the water in each test case … but it was on the order of the amount that could easily settle into my tank over several weeks. This experiment clearly showed this transport mechanism as a viable ammonia source.
Next, to confirm the dryer sheets as the source of the ammonia on the lint specifically, I took a small fragment of a Bounce dryer sheet and soaked it for a few minutes in saltwater and again found high TA at 0.8 ppm (see Figure 4). It appeared to be at least in part the Bounce fabric softener sheets in use with the dryer. Note too that dryer sheets transfer a chemical coating to fabric through the sheet being heated. In this case the sheet fragment was not heated which would likely transfer far less QAC out of the sheet and into the saltwater as compared to what would occur in the dryer. I then did some research on fabric softeners and I found that almost all of them - as well as many many other things in our home environment - contain Quaternary Ammonia Compounds (QACs). I looked up the “smart label” data (detailed listing of ingredients) online for the Bounce sheets and one of its primary ingredients is Dialkylester Dimethyl Ammonium Methosulfate. Moreover there are some 52 other compounds that create “fragrance” in these Bounce sheets. Yikes!!! I'm not a chemist … but they all have scary names that only chemists can pronounce ... I would think no one would want any of them in their tank! I sure don't.
Then as a follow up test, I extensively cleaned the lint trap of the dryer and ran 5-6 loads of laundry with NO dryer sheets but still using typical laundry detergent in the wash cycle prior to the drying cycle. I then collected this lint from the lint trap and again mixed with my saltwater and tested TA. I was surprised to get almost zero TA at 0.1! Note I was not able to perfectly clean the entire inside of the dryer and expect over time that this would diminish even further. The prior lint when using dryer sheets appears to have contributed all (or at least most) of the TA in the prior readings. Now that I had removed the use of Bounce dryer sheets the resulting lint in the trap (and therefore likely the lint in the air) were clear of ammonia.

Figure 3: Total Ammonia readings after cleaning lint trap with no Bounce sheets and dust/lint from room. The dust/lint vial was collected from the tops of picture frames in the room - this dust settled when Bounce sheets were still in use prior to lint trap cleaning. RODI control (TA=0ppm). Lint from dryer trap after cleaning (TA=0.1 ppm). Dust/lint in room (TA=0.8 ppm)
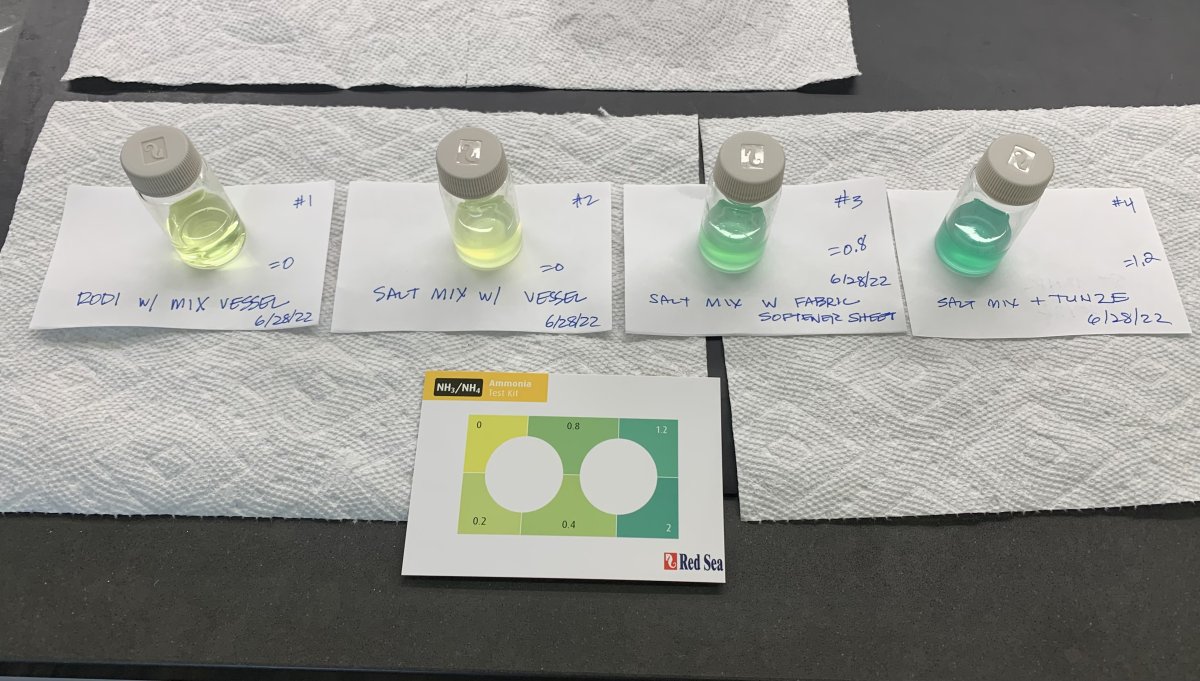
Figure 4: RODI and salt water mix control (TA=0 ppm). Fabric softener fragment (TA=0.8 ppm). Tunze Care Panes cleaner (TA=1.2 ppm). Note that specific magnitudes were not controlled for, rather these tests showed the presence or absence of ammonia indication only.
In addition to the possible introduction of ammonia into the system, there also appears to be a lot of other reasons that QACS are not good for aquatic life and I have found many technical papers that list QACs as such. I also recently spent (too many) hours reading about the infamous Vibrant situation where polyQACs are apparently involved as well. It is clear that QACs are bad in many ways.
At this time it seems very feasible that QACs in signifiant quantities were transported into the tank. The only way I can positively confirm this beyond a shadow of doubt is to directly test for them. I'm looking into these testing options and any ideas on reasonably priced ways to conduct them are welcome. It is not clear if online services will/can test for QACs.
But in the meantime I want to get any comments from the community on this 12-week adventure I have been on. Am I missing anything here? Also I would love to get some expert QAC chemistry explanations on the possible mechanisms that would be at work here and if high(er) ammonia readings would be consistent with the presence of QACs.
At the same time I have noticed a large cluster of unexplainable system crashes and stalled cycles in the online forums that seemingly go unresolved. User brandon429 seems to have great passion on this topic and he has done a great deal of work in this area. He even has come up with an approach to confirm the presence of a cycle by looking for movement in the readings - effectively by removing/ignoring any constant offset of ammonia. It indeed is a very clever approach and would be consistent with accounting for a positive interference in the testing methodology (possibly QACs) that are offsetting results in a reliable deterministic fashion. The fact that fish and coral are living/thriving in these ultra high ammonia cases makes one conclude that the tests are clearly wrong. But maybe it is more nuanced than that ... maybe the tests are just being used in an environment which have positive interference. Are QACs possibly involved more than we realize in these situations? And this brings me to the last point/idea.
If the expert community thinks that indeed QACs in my case are responsible for my high ammonia readings then either the QACs 1) destroyed the bacteria that was needed, 2) acted as a positive interference in the testing, or 3) introduced ammonia into the tank. Or possibly all of them in various combinations? But regardless, under any of these cases - if an aquarist sees unexpected ammonia spikes - or the presence of baseline ammonia in the system that is otherwise unexplainable - does this represent a possible surrogate indicator for QACs (or other interferences)? A so called "Canary in the Coal Mine" for the possible introduction or build up of QACs in our tanks? If so then follow on testing would be warranted which also suggests the need to have affordable ways to measure QACs in our tanks at the concentrations of interest.
Although the last 3 months have been frustrating as I attempt to cycle my first tank - I’m super excited to be entering the hobby. I have always loved technical challenges … and clearly this hobby has them! Can't wait to actually buy some coral and fish some day!
I am having problems fishless cycling my coral quarantine tank - and it seems that I am possibly in a stalled cycle state. I have tried various substrates over the past 12 weeks and have monitored tank parameters almost daily. I have narrowed several things down and now suspect that Quaternary Ammonia Compounds (QACs) from fabric softening dryer sheets (standard Bounce Outdoor Fresh scented) have been transported into the tank through lint settling in the air. I want to get a read from the expert community if I’m on the right track here and I also want to understand the chemical mechanisms that could be at work.
Here are the details:
Tank Setup
My coral QT tank is located with my salt water mixing station in a laundry room (Figure 1). This tank is a 10 gal Fiji Cube (7.6 gal of actual water volume) and is bare bottom with no rock. Initially upon filling the tank I used 18 MarinPure Biomedia 1.5” Spheres as a substrate, Brightwell MicroBacter QuickCycle as my ammonia source, and Birghtwell MicroBacter Start XLM as my nitrifying bacteria source. My salt mix is Red Sea Coral Pro and I use all Red Sea test kits. I should also note that I use a 7-stage BRS RODI filter system and have confirmed zero TDS in my RODI output water. I also verified less than 1 ppb Total Chlorine with Hanna Ultra Low Range Checker in my RODI water. (NOTE: My tap water is 40 ppb total chlorine. My water utility is clearly using chloramines).Figure 1: The Coral QT tank in Laundry Room.
First Tank Cycle Attempt
I was hopeful for a fast 10-day cycle but was also aware that small bare bottom QT tanks are notorious for needing more time and patience due to a lack of suitable surface area for the bacteria to take hold. It took nearly a month to cycle the tank. I took detailed readings of total ammonia, nitrite, and nitrate daily (see Figure 2 below). The shape of these curves for the most part tracked “typical” production and decay trends. At the completion of this month long process the total ammonia was reduced from 1.6 ppm to very near (but not quite) zero total ammonia (< 0.1 ppm), nitrite had spiked at 0.2 ppm and then disappeared, and I was at 2 ppm nitrate. After almost a week at this non-zero reading of < 0.1 ppm, I assumed that I was having difficulty reading zero precisely with my test kit. It seemed like the tank had for the most part cycled, but I wanted to be sure and test that before introducing livestock.Did the Tank Cycle?
I wanted to double check and test the cycled tank by simulating some bioload before adding any livestock. I added a small amount of QuickCycle (estimated to be a typical couple of days of bioload for a fish system of this size). This took the TA to 0.8 ppm and I expected to see it consumed within about a 24-48 hour period. Unfortunately, there was little to no response at all - I then kept supplementing with MicroBacter Start XLM and it brought it down over the course of a couple weeks. During this time it seemed like it was the bacteria dosing in the water column that was doing the work. It finally settled again at about 0.1 ppm this time a little higher than before. In retrospect having it spike to 0.8 ppm was a bit high for a 2 day bioload ... but even in this case it didn't feel like it should have taken 2 weeks with multiple supplemental bacteria doses to bring ammonia back down (these dosing times are not denoted in Figure 2). It didn't seem like the tank had cycled and I was worried that the reason the ammonia ultimately came down was due to my continued dosing of the bacteria and not because I had established a suitable microbial biome.Attempting a Re-cycle with more Substrate
Next I decided to do a 50% water change and then I removed my substrate (18 MarinePure ceramic bioballs) from the sump area to move into display area where I could visually examine if biofilm was forming on the media. The ceramic bioballs were in a filter mesh bag for easy removal. At this point I was shocked to find a strong biofilm coating on the entire outside of the bag. This is a good sign - right? But then I placed it in the display area and it floated! The entire bag floated with ceramic balls. The biofilm on the outside of the bag was so well formed that it was encasing the bag and sealing gas inside of it and it actually floated the bag. This was surprising to me (can someone else confirm ever seeing this?). At this point I realized that I was clearly not getting much flow into the bag where the balls were and because it was sealed off it likely was a pretty anaerobic environment. So the tank was not benefiting from the massive surface area of the media itself to grow beneficial bacteria. I started wondering if possibly I had too low of surface area for the beneficial bacteria to cycle my tank. At this point I decided to switch to the Brightwell recommended Lattice Nitraz and much more porous filter bags. I put in 2-3 times the recommended amount of Lattice media and filled the rest of my sump with 20 plastic bio balls (older technology but many experts swear by it for aerobic uses). I introduced them slowly over many days as I removed the MarinPure bio media balls and agitated them before removal to slough off as much beneficial bacteria into the water column as possible. I also re-dosed MicroBacter Start XLM all over again in an attempt to reseed the beneficial bacteria in the other media. At this point it seemed that I had plenty of the bacteria present in my tank.I then added a couple of drops of QuicCycle to simulate a bioload to see if the tank would consume the ammonia. TA immediately spiked this time at 0.6 ppm and stayed there for over a week. Then it began going up! Wait! TA going up with no livestock? Something was wrong here. Over the next few days the TA reached 0.8 ppm and was there for several days. I continued to add supplemental bacteria and it did then go down over the next several days and this time the TA leveled off at 0.3 ppm - well above zero. At this point it felt like once again I had a stalled tank. I still had ammonia. I had helped bring it down with additional bacteria dosing but It was (and is still) stuck at TA of 0.3 ppm.
Figure 2: Cycling Data: Ammonia, Nitrite, and Nitrate over 12 weeks. Initial cycle and two subsequent stalled tests shown. There are a couple noisy/erroneous readings that are apparent in the data). Times of bacteria dosing not shown.
Finding the Problem - The Investigation into QACs
At this point I concluded that I either a) I had another ammonia source in my tank, or b) my TA readings were wrong. First, in order to investigate I wanted to eliminate faulty readings. I made up several precise salt water batches and through several checks of my testing kits (I have multiple Red Sea kits) I got very consistently zero TA readings on these batches across more than one kit. I should also note that the other previous non-zero TA tests described above were reproducible - in many cases I double tested them. I convinced myself that the test kits and my use of them were not at issue and I was able to test zero TA with them.I then turned my attention to possible ammonia sources and thought that it could be introduced through the air. I first looked through the tank for any large bugs or any other organics that might have fallen into the tank but I found none. Recall, that this tank is in a laundry room and I next focused on this and especially on the chemicals in the room. The laundry detergents in use (sometimes containing bleach) are kept far away from the tank - but is it possible that bleach could transport through the air? This seemed unlikely but I wanted to rule it out. I also considered the fabric softener dryer sheets in use as there is a lot of dust (i.e. lint) that forms on horizontal surfaces in the room. (Note: There are a couple existing discussions on R2R about tanks in laundry rooms but there is nothing conclusive in those threads on the associated risks.)
I purchased the Total Chlorine ULR Hanna checker and got 5 ppb TC in the tank. This was higher than my target of keeping it below 2 ppb as I found some articles suggest this level for marine environments. Recall, I get 1 ppb TC out of my 7-stage RODI so the in-tank readings showed additional TC over the source RODI water. This 5 ppb TC reading was concerning since it was clearly above the suggested safe levels. It was (and still is) not clear what impacts this has on the tank, but my focus moved quickly to fabric softeners since what I found was even more concerning.
I mixed up a precise batch of saltwater and confirmed zero TA in it. I then pulled a small amount of lint from the lint trap in the dryer, soaked it in 100 ml of this saltwater for a few minutes and then tested for ammonia. I got high levels of TA at nearly 2 ppm (this test not shown in figures). I then went around the room and collected small amounts of dust (with a wet finger) from the tops of picture frames on the walls and transferred this dust/lint into 100 ml of salt water … this too tested high for TA at 0.8 ppm (shown in Figure 3). This testing was to discern the presence or lack thereof of ammonia in the lint/dust and it showed that it was clearly present and at levels that surprised me relative to the small amounts of lint that I collected. I did not control for the precise amount of lint/dust dissolved into the water in each test case … but it was on the order of the amount that could easily settle into my tank over several weeks. This experiment clearly showed this transport mechanism as a viable ammonia source.
Next, to confirm the dryer sheets as the source of the ammonia on the lint specifically, I took a small fragment of a Bounce dryer sheet and soaked it for a few minutes in saltwater and again found high TA at 0.8 ppm (see Figure 4). It appeared to be at least in part the Bounce fabric softener sheets in use with the dryer. Note too that dryer sheets transfer a chemical coating to fabric through the sheet being heated. In this case the sheet fragment was not heated which would likely transfer far less QAC out of the sheet and into the saltwater as compared to what would occur in the dryer. I then did some research on fabric softeners and I found that almost all of them - as well as many many other things in our home environment - contain Quaternary Ammonia Compounds (QACs). I looked up the “smart label” data (detailed listing of ingredients) online for the Bounce sheets and one of its primary ingredients is Dialkylester Dimethyl Ammonium Methosulfate. Moreover there are some 52 other compounds that create “fragrance” in these Bounce sheets. Yikes!!! I'm not a chemist … but they all have scary names that only chemists can pronounce ... I would think no one would want any of them in their tank! I sure don't.
Then as a follow up test, I extensively cleaned the lint trap of the dryer and ran 5-6 loads of laundry with NO dryer sheets but still using typical laundry detergent in the wash cycle prior to the drying cycle. I then collected this lint from the lint trap and again mixed with my saltwater and tested TA. I was surprised to get almost zero TA at 0.1! Note I was not able to perfectly clean the entire inside of the dryer and expect over time that this would diminish even further. The prior lint when using dryer sheets appears to have contributed all (or at least most) of the TA in the prior readings. Now that I had removed the use of Bounce dryer sheets the resulting lint in the trap (and therefore likely the lint in the air) were clear of ammonia.
Figure 3: Total Ammonia readings after cleaning lint trap with no Bounce sheets and dust/lint from room. The dust/lint vial was collected from the tops of picture frames in the room - this dust settled when Bounce sheets were still in use prior to lint trap cleaning. RODI control (TA=0ppm). Lint from dryer trap after cleaning (TA=0.1 ppm). Dust/lint in room (TA=0.8 ppm)
Figure 4: RODI and salt water mix control (TA=0 ppm). Fabric softener fragment (TA=0.8 ppm). Tunze Care Panes cleaner (TA=1.2 ppm). Note that specific magnitudes were not controlled for, rather these tests showed the presence or absence of ammonia indication only.
QACs in Tank
Based on these tests I am currently concluding that a significant amount of lint over time (in this case many weeks) was transported into my QT tank by settling through the air. Furthermore, during the time that Bounce dryer sheets were being used in the dryer (almost daily over many weeks) this lint was coated with significant amounts of QACs. It seems that initially the tank may have cycled but during and towards the end of that time enough QAC was accumulating that I lost the cycle - or at a minimum the cycle was fine but the tests possibly wrongly indicated the perpetual presence of ammonia. Over the last two months the baseline ammonia of the tank has continued to increase and it was not until I removed Bounce sheets that it seemingly has plateaued now at TA 0.3 ppm. Coincidence? Possibly. But at this point I have no better working hypothesis for the TA readings in my tank.In addition to the possible introduction of ammonia into the system, there also appears to be a lot of other reasons that QACS are not good for aquatic life and I have found many technical papers that list QACs as such. I also recently spent (too many) hours reading about the infamous Vibrant situation where polyQACs are apparently involved as well. It is clear that QACs are bad in many ways.
At this time it seems very feasible that QACs in signifiant quantities were transported into the tank. The only way I can positively confirm this beyond a shadow of doubt is to directly test for them. I'm looking into these testing options and any ideas on reasonably priced ways to conduct them are welcome. It is not clear if online services will/can test for QACs.
Current Course of Action
My current course of action at this point (pending better advice) is to do a 100% water change, complete cleaning, and start again with NO bounce sheets in use in the laundry room. Furthermore, as a precautionary measure, I am planning to add a desktop air purifier to the room near the tank to further extract lint/dust from the air.But in the meantime I want to get any comments from the community on this 12-week adventure I have been on. Am I missing anything here? Also I would love to get some expert QAC chemistry explanations on the possible mechanisms that would be at work here and if high(er) ammonia readings would be consistent with the presence of QACs.
Questions
- Does the conclusions that QACs are likely the culprit for high TA readings and the inability to cycle make sense?
- Is it possible that the QACs are just a positive interference in the salicylate-based test kit? Like iron or copper typically are? For example, is it possible the QAC R groups are stable in the tank but are broken down during the testing methodology (introduction of reagents) and thus depending on pH convert into NH3 and NH4+ only in the test vial?
- Is there really ammonia being introduced? I read that QACs (at least on some test strips) do not register in TA (but those test strips may not be salicylate-like). Is it possible that only ammonium NH4+ and not free ammonia NH3 is being introduced? And would that even really matter since it would eventually be present for conversion? Note: I have a Seachem Ammonia badge in the tank that always seems to register lower free ammonia than the Red Sea tests would indicate (after pH and temp correction lookup). Admittedly a visual badge of this nature is not super precise and I can not confirm if these badges directly measures free ammonia or if it is simply inferred on the badge through actually measuring total ammonia. This is an anecdotal observation that keeps my attention and makes me think positive interference is a possibility within the test kit.
- Is it that the QAC level will not allow beneficial bacteria to survive or at least at a minimum not allow it to thrive to the extent needed for ammonia reduction?
- When I restart/recycle the tank would it make the most sense to dispose of all Lattice Nitraz, plastic bioballs, and MarinPure spheres? It seems like it is possible that QACs as a cation could possibly remain attached to the medium?
- I looked into inexpensive test strips where I could test for QACs. The food industry uses these, but I could not find any confirmation that they are accurate in salt water at the pH levels we operate in and it is not clear to me what precision I would even need to assess reef safety. Any insights here are welcome.
- My total chlorine did go up from 1 ppb to 5 ppb within the tank. Would QACs possibly do this? Or do I have another possible contaminating source of Chlorine? What levels of Total Chlorine would you consider bad in a reef environment?
Additional Observations
- I am leaving the tank up for another 5 weeks as I go on a long vacation with an ATO operating and some remote monitoring capability. I am curious to see if there is any recovery at all of this situation. I expect that the QACs will not degrade on their own and I will continue to see the non-zero TA readings after 5 weeks. But the point here is that I still have access to the current water chemistry in the tank if anyone thinks I could conduct further testing on it.
- I would have loved to see a Seneye in tank during this process. I'm thinking about getting one when I restart and recycle the tank. I suppose it could be useful to me later as I take fish quarantine tanks up and down and possible hospital tanks.
- I have also been dosing magnesium, alk, and calcium via Red Sea Foundation elements. I have been pulling my hair out with low alk readings regardless of how much Red Sea Foundation B I dose. I have even conducted a pause and then ramped back up slowly as Randy Holmes-Farley suggests in one of his online essays. The original Coral Pro salt water mix was confirmed to be 11.5 dKH when the tank was filled as well as on the subsequent 50% water change. This tank quickly goes to 6-6.3 dKH regardless of my attempts at correcting it. I have stopped trying as I just continue to get calcium carbonate precipitate and have had my return pump seize. My Mg is staying at 1350-1400 ppm and Ca at 420-460 ppm …. and notably the Ca is getting consumed (albeit more slowly) as the CaCO3 forms. Also, I have read Randy H-F’s explanation of Mg(OH)2 formation as a cloud when dosing. I dose into strong flow to limit the amount of time there is a high pH environment at work … but right after the magnesium hydroxide cloud starts to dissipate (about 2 secs) I see a clear precipitate form in the water column where the cloud was … it looks like snow (some fairly large flakes). This swirls around for a while and I am not sure if it goes back into solution or settles. What is this? I didn't think CaCO3 could form that fast in the water column itself. This is an odd effect that I could not find an explanation for. At this point I wonder if it could be related to the presence of the QACs? Is the inability to keep higher alk levels possibly consistent with the presence of QACs?
- I have had a Neptune Apex in the tank for a couple of weeks. The ORP has never exceeded 170-185 mV which is very low. I know that many people discourage paying too much attention to this parameter, but would low ORP be consistent with the presence of QACs?
- I use Tunze Care Panes to clean my aquarium glass. I'm very cautious not to get any of it into the tank. But I did do a test on a small amount of it on a rag and then soaked in salt water and it resulted in incredibly high TA readings. I had several email exchanges with the company and their product engineers. They even provided me with the MSDS and assured me that all ingredients are "natural" botanical oils, water, and alcohol and that it is impossible for any ammonia or QACs to be introduced from their product. Their only suggestion is that some ingredient in their product, while safe, could be a positive interference on the salicylate method.
Ramifications and Ideas Going Forward
I'm surprised to find how prevalent QACs are and how (seemingly) easily they can make their way into our tanks. Moreover, there have recently been researchers expressing concern about the increased use of disinfectants of all kinds due to COVID-19. These disinfectants often times contain QACs. Also, there are supposedly more than 200 commercial product categories that contain QACs - air fresheners, detergents, disinfectants, fabric softeners, etc. There are many potential sources: A cleaner or air freshener sprayed in the room? Disinfecting your hands with anti microbial lotions/cleaners and then shortly after working in the tank? Fabric softener laden lint in the air? The list goes on.At the same time I have noticed a large cluster of unexplainable system crashes and stalled cycles in the online forums that seemingly go unresolved. User brandon429 seems to have great passion on this topic and he has done a great deal of work in this area. He even has come up with an approach to confirm the presence of a cycle by looking for movement in the readings - effectively by removing/ignoring any constant offset of ammonia. It indeed is a very clever approach and would be consistent with accounting for a positive interference in the testing methodology (possibly QACs) that are offsetting results in a reliable deterministic fashion. The fact that fish and coral are living/thriving in these ultra high ammonia cases makes one conclude that the tests are clearly wrong. But maybe it is more nuanced than that ... maybe the tests are just being used in an environment which have positive interference. Are QACs possibly involved more than we realize in these situations? And this brings me to the last point/idea.
If the expert community thinks that indeed QACs in my case are responsible for my high ammonia readings then either the QACs 1) destroyed the bacteria that was needed, 2) acted as a positive interference in the testing, or 3) introduced ammonia into the tank. Or possibly all of them in various combinations? But regardless, under any of these cases - if an aquarist sees unexpected ammonia spikes - or the presence of baseline ammonia in the system that is otherwise unexplainable - does this represent a possible surrogate indicator for QACs (or other interferences)? A so called "Canary in the Coal Mine" for the possible introduction or build up of QACs in our tanks? If so then follow on testing would be warranted which also suggests the need to have affordable ways to measure QACs in our tanks at the concentrations of interest.
Closing
Thanks to those who read this far! Admittedly, this was a VERY long (first) post here on the forum and hopefully not too overly detailed. I have been on this adventure for a few months now and wanted to provide as much detail as possible and then get the community's thoughts.Although the last 3 months have been frustrating as I attempt to cycle my first tank - I’m super excited to be entering the hobby. I have always loved technical challenges … and clearly this hobby has them! Can't wait to actually buy some coral and fish some day!






















