- Joined
- Sep 7, 2017
- Messages
- 735
- Reaction score
- 916
Hello all!
I have a weird one, I've been dosing Kalk for a short time and when I'm home, with the dog and me breathing like mad all over the house it works fabulous, but when I leave for vacation I find that I need to tipple the ALK 2 part dosing to not even quite keep up. I know Kalk requires CO2 for the Alk part, is it possible that with no breathing it doesn't work as welll?
Here's the details:
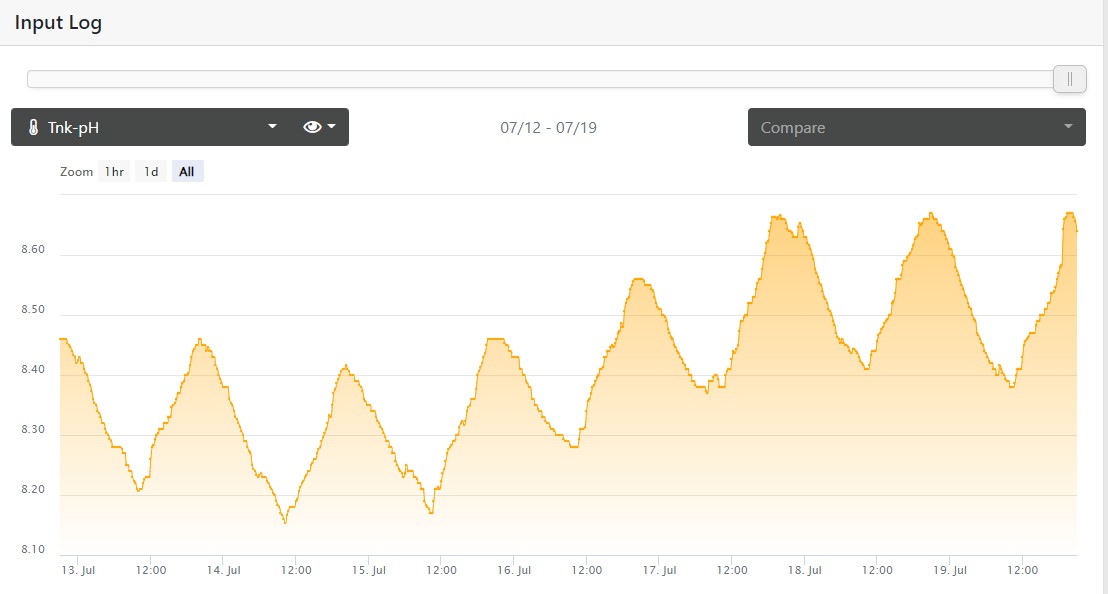
My PH when I'm home on the left, I was gone on a work trip on the right, this probe was calibrated this month:
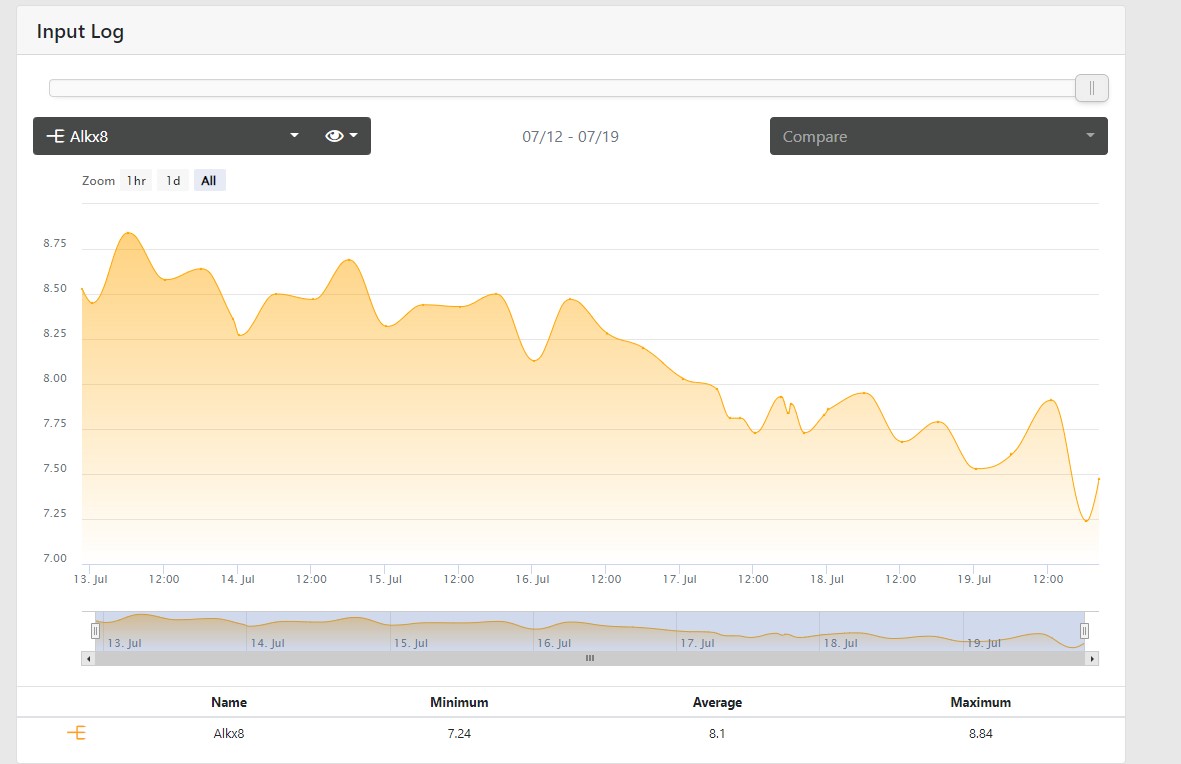
Normally I dose 80 ML of Core 7 Alk daily, when I'm gone I am pushing that to over 250 ML and the ALK still falls,.. Here is that graph:
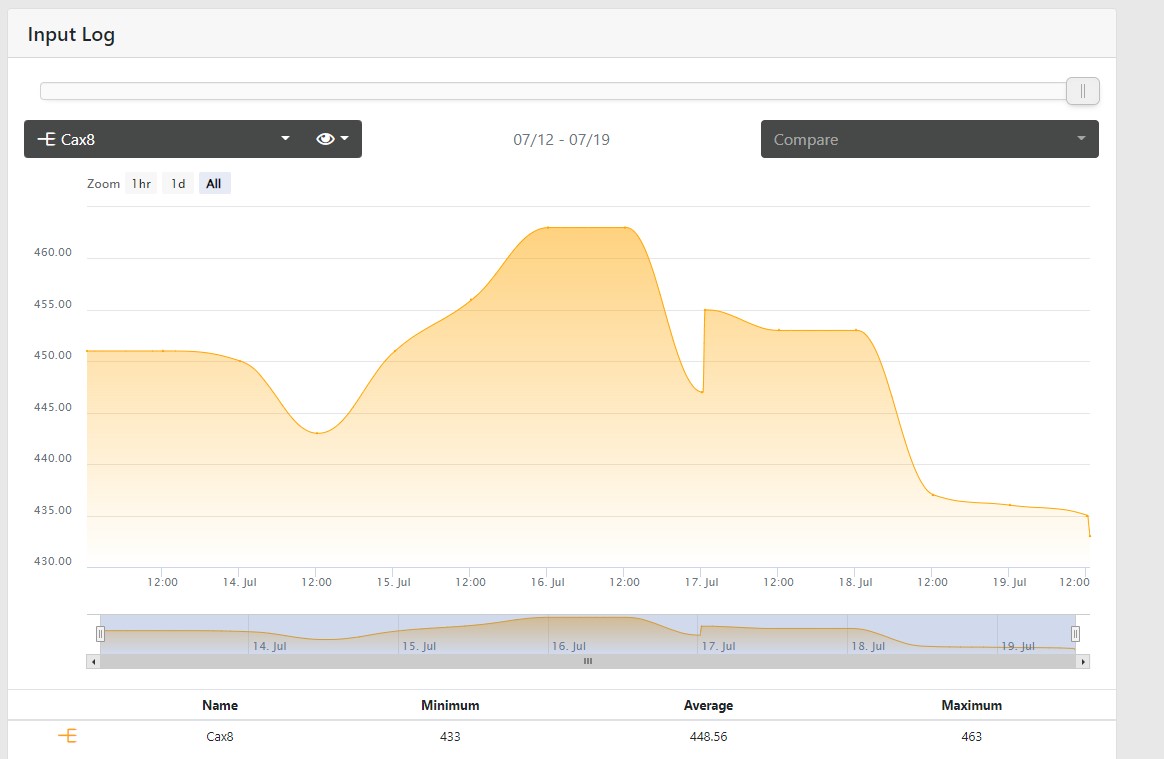
At first I thought that my growth might be going through the roof when I travel, and it might, but my CA doesn't seem to be effected as dramatically, though it is a little bit. I did not change CA dosing at all:
What do you think? Could it be possible that I don't have the CO2 to make the ALK part of Kalk work when I'm gone? Or do you think the super high PH made my corals grow like crazy and I should run a skimmer airline outside to have that all the time?
Thank you!
Whiskey
I have a weird one, I've been dosing Kalk for a short time and when I'm home, with the dog and me breathing like mad all over the house it works fabulous, but when I leave for vacation I find that I need to tipple the ALK 2 part dosing to not even quite keep up. I know Kalk requires CO2 for the Alk part, is it possible that with no breathing it doesn't work as welll?
Here's the details:
My PH when I'm home on the left, I was gone on a work trip on the right, this probe was calibrated this month:
Normally I dose 80 ML of Core 7 Alk daily, when I'm gone I am pushing that to over 250 ML and the ALK still falls,.. Here is that graph:
At first I thought that my growth might be going through the roof when I travel, and it might, but my CA doesn't seem to be effected as dramatically, though it is a little bit. I did not change CA dosing at all:
What do you think? Could it be possible that I don't have the CO2 to make the ALK part of Kalk work when I'm gone? Or do you think the super high PH made my corals grow like crazy and I should run a skimmer airline outside to have that all the time?
Thank you!
Whiskey
















