also must add: I gotta see that nh3 totally stuck on a digital meter to be 100% bought in its dead. ya'lls known api skills have me at 99.9% tho 
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Does my decade old sand bed actually nitrify? Who eats Ammonia in our tanks?
- Thread starter taricha
- Start date
- Tagged users None
Or you could have a contained environment outside of the display like Donovan's Nitrate Destroyer.(If I had high NO3, I would want anaerobic space where denitrification might be accelerated)
For what it's worth I'm pretty neutral on sand but my wife likes the look and I want it for nassarius snails, gobies, and wrasses. I saw a mature bare-bottom softie tank at a "local" LFS and it looked really cool with the coralline algae covering the glass.
- Joined
- May 22, 2016
- Messages
- 6,526
- Reaction score
- 10,058
I couldn't help myself so I set up three 5 gal tanks...

Each tank got Instant Ocean water, 500mL Caribsea aragonite dry sand, and low quality "live" rock from Petco. (The rocks had all been kept in a pile in the same tank. I imagine they are pretty similar). Each tank got the minimum recommended dose of Biospira, and were left to circulate with the lights off.
Tank 1 is left to "cure", and gets no feeding except the few tenths ppm ammonia in I.O. and whatever organics comes from the rock.
Tank 2 and 3 were traditionally cycled using a schedule based on Dr. Tim's fishless cycling. Day 1, 3 and 6 the tanks got the same 1 to 1.5ppm ammonia drops.
Day 8 ammonia was zero in all 3 tanks.
Day 9 I added ammonia to each tank to test the consumption rates...

Tank 1 that was just left to "cure" did great. Data in blue, it consumed about 0.5ppm TAN/day.
Tanks 2 & 3 that were actually cycled were so fast they depleted my first dose and I had to re-dose ammonia to get a measure on the consumption rate.
Data in Red and Green, they are both showing consumption of around ~1.7ppm TAN per day.
In case anyone else wanted to visualize how much the nitrification ability is expanded during a normal cycling routine. Tanks ended the 9 day cycle with 3 to 4x the nitrification rate of a tank that was given the same bacteria but not fed.
Each tank got Instant Ocean water, 500mL Caribsea aragonite dry sand, and low quality "live" rock from Petco. (The rocks had all been kept in a pile in the same tank. I imagine they are pretty similar). Each tank got the minimum recommended dose of Biospira, and were left to circulate with the lights off.
Tank 1 is left to "cure", and gets no feeding except the few tenths ppm ammonia in I.O. and whatever organics comes from the rock.
Tank 2 and 3 were traditionally cycled using a schedule based on Dr. Tim's fishless cycling. Day 1, 3 and 6 the tanks got the same 1 to 1.5ppm ammonia drops.
Day 8 ammonia was zero in all 3 tanks.
Day 9 I added ammonia to each tank to test the consumption rates...
Tank 1 that was just left to "cure" did great. Data in blue, it consumed about 0.5ppm TAN/day.
Tanks 2 & 3 that were actually cycled were so fast they depleted my first dose and I had to re-dose ammonia to get a measure on the consumption rate.
Data in Red and Green, they are both showing consumption of around ~1.7ppm TAN per day.
In case anyone else wanted to visualize how much the nitrification ability is expanded during a normal cycling routine. Tanks ended the 9 day cycle with 3 to 4x the nitrification rate of a tank that was given the same bacteria but not fed.
Last edited:
T I can’t wait to see them tested after a full water change
not that it’s any great finding to see but just a little twist up as it seems you’ve approximated the flow and surface area evenly in all tests
after a full wc do they all streamline or does the boosted ones still outpace, this is juicy surface area physics to see
wonder if suspension cycling (swirling suspended unattached nitrifiers destined to be sinked or skimmed) is helping the boost and removal at the faster rate, will a water change even out the performance
not that it’s any great finding to see but just a little twist up as it seems you’ve approximated the flow and surface area evenly in all tests
after a full wc do they all streamline or does the boosted ones still outpace, this is juicy surface area physics to see
wonder if suspension cycling (swirling suspended unattached nitrifiers destined to be sinked or skimmed) is helping the boost and removal at the faster rate, will a water change even out the performance
Last edited:
T what's also very important is that your non dosed rocks didn't spike, stall, or act outside the provisions from a 1932 wastewater cycling chart. that's a big deal a big big deal
it takes your testing skills and alterations to see it, res publica cannot discern this using their common instructions and test kits.
it takes your unique testing alterations and chemistry skills to make me believe anything about free ammonia not coming from a digital readout calibrated to show alternate readings in other known settings
the degree of cycle ability between the two systems specifically means all will carry the same bioload. you can't pack enough fish into the undosed one to cause an nh3 death spike because that'd be inputting an unreasonable fish load for that tank.
absolutely ANY common bioload we'd select to reef with in those small nanos is equally carried though one tank or two moves ammonia faster than another.
they all have the same collective ability, and if we upscale to larger tanks that's just more surface area and dilution to allow for again similar initial bioloading. this is scalable physics in my opinion. all the way down to half gallon reefs or seventeen thousand gallon reefs
I think the new info coming to light about post-cycle nh3 regulation is going to show that stark variations in tank bioloading still result in the same conversion rate, half gallon pico to seventeen thousand gallon home tank with 200+ fish. its all in the presentation of the surface area to the wastewater (can't squirrel away the surface area in a sump only, direct immediate contact when emitted in the display up top is powerful) it is NOT about the bac we've paid for redundantly. those are short term gains soon equalized by common tank maintenance.
nature is equalizing things in a shocking way, I truly think future results will show some degree of this scalability.
I bet nobody's testing for cycling rules invalidates what an ammonia line does from any typical cycling chart pick the site or book, they're all about the same. that's so profound in my opinion...redundant bottle bac sales machine depends on folks doubting those cycling charts, and they need something to buy for peace of mind.
it takes your testing skills and alterations to see it, res publica cannot discern this using their common instructions and test kits.
it takes your unique testing alterations and chemistry skills to make me believe anything about free ammonia not coming from a digital readout calibrated to show alternate readings in other known settings
the degree of cycle ability between the two systems specifically means all will carry the same bioload. you can't pack enough fish into the undosed one to cause an nh3 death spike because that'd be inputting an unreasonable fish load for that tank.
absolutely ANY common bioload we'd select to reef with in those small nanos is equally carried though one tank or two moves ammonia faster than another.
they all have the same collective ability, and if we upscale to larger tanks that's just more surface area and dilution to allow for again similar initial bioloading. this is scalable physics in my opinion. all the way down to half gallon reefs or seventeen thousand gallon reefs
I think the new info coming to light about post-cycle nh3 regulation is going to show that stark variations in tank bioloading still result in the same conversion rate, half gallon pico to seventeen thousand gallon home tank with 200+ fish. its all in the presentation of the surface area to the wastewater (can't squirrel away the surface area in a sump only, direct immediate contact when emitted in the display up top is powerful) it is NOT about the bac we've paid for redundantly. those are short term gains soon equalized by common tank maintenance.
nature is equalizing things in a shocking way, I truly think future results will show some degree of this scalability.
I bet nobody's testing for cycling rules invalidates what an ammonia line does from any typical cycling chart pick the site or book, they're all about the same. that's so profound in my opinion...redundant bottle bac sales machine depends on folks doubting those cycling charts, and they need something to buy for peace of mind.
- Joined
- May 22, 2016
- Messages
- 6,526
- Reaction score
- 10,058
I have an odd respect for your persistence with an idea that no one else in the hobby thinks is important (nitrification in the water after a cycle).wonder if suspension cycling (swirling suspended unattached nitrifiers destined to be sinked or skimmed) is helping the boost and removal at the faster rate, will a water change even out the performance
You're right it's worth checking, because I have no filtration or skimming so if there were planktonic nitrifiers, they would have no obstacles.
I got you covered. I'm pulling a 100mL sample of just water and another sample of water + 2% sand from a cycled tank. We'll see if the water is active, or if all the activity immediately after a cycle is in the sand.
also want to add where we cycled an entire 200 gallon tank in 20 days by using only reef water:

 www.reef2reef.com
that's meant to skew your findings lol
www.reef2reef.com
that's meant to skew your findings lol
hey if I could change one thing in that test we did it would be to black out the new tank in 100% darkness to rule out plant uptake in the outcome.

Connecting Established Reef To New Tank
EDIT: In conclusion, if using the method I did - connecting an established reef to a new system with dry rock (clean), I recommend the following: 1. Dose your new tank prior to connecting to your old tank with NO3 and PO4 enough to raise to your established tank levels. 2. Take your skimmer...
 www.reef2reef.com
www.reef2reef.com
hey if I could change one thing in that test we did it would be to black out the new tank in 100% darkness to rule out plant uptake in the outcome.
Last edited:
- Joined
- May 22, 2016
- Messages
- 6,526
- Reaction score
- 10,058
wonder if suspension cycling (swirling suspended unattached nitrifiers destined to be sinked or skimmed) is helping the boost and removal at the faster rate, will a water change even out the performance
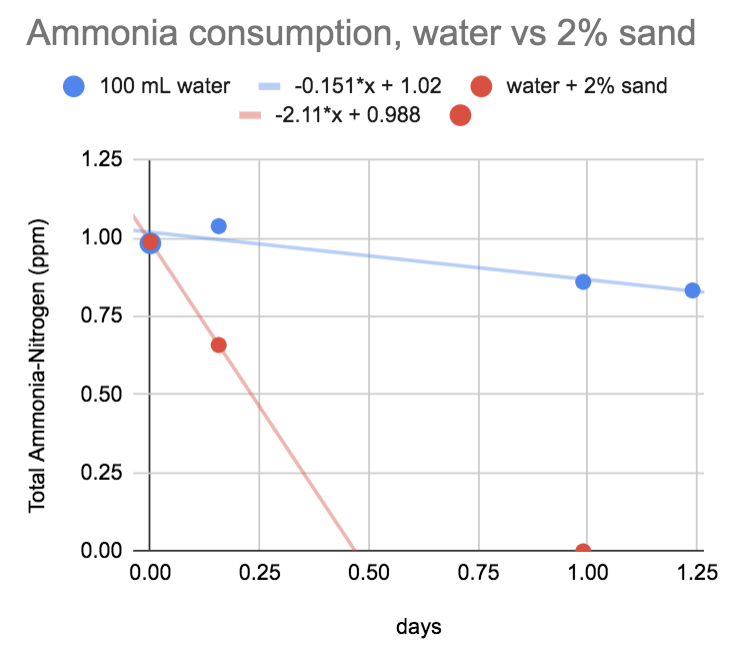
So here's the results to this
ammonia added to one of the cycled tanks.I got you covered. I'm pulling a 100mL sample of just water and another sample of water + 2% sand from a cycled tank. We'll see if the water is active, or if all the activity immediately after a cycle is in the sand.
100mL of water-only in one sample
100mL of water with 2% (2mL) sand in the other sample.
samples on orbital shaker at 70rpm in the dark...
The sample with the newly cycled sand is processing at a rate of ~2ppm ammonia-nitrogen per day (about the same rate as the cycled tank the sample was pulled from).
The water only - less than 1/10 of that, ~0.15 ppm per day. It's probably not zero, but I can't really nail it down 100% because the water was filled with NO2/NO3 before I started, so I can be definitive and show the corresponding increase in NO2/NO3.)
I can say from this that no one should ever worry about losing nitrification capacity from doing a 90% water change after a completed cycle, you would lose an un-noticeable amount of nitrification.
(Unless you cycled with a product that isn't a chemoautotrophic nitrifier. Then all bets are off. Those heterotrophs could be anywhere.)
This also ties up the loose end for me: That the protocol I used (a few % sand in 100mL jar on an orbital shaker at 70rpm) is actually capable of detecting high nitrification rates of several ppm per day.
So those samples from my tank and the LFS that only nitrified a couple of tenths ppm per day, they simply didn't have many nitrifiers.
A single dose of biospira + worst possible live rock + dry sand + 9 day cycle = more than 10x the nitrification rate of my 10yr old sand bed.
A single dose of biospira + worst possible live rock + dry sand + 9 day cycle = more than 10x the nitrification rate of my 10yr old sand bed.
You have one lazy old sand bed!
- Joined
- Sep 21, 2018
- Messages
- 6,649
- Reaction score
- 7,136
A point of interest. The biofilm that forms on glass slides in my system after 15 days do not oxidize ammonia but coat glass slides with aragonite and in five days the glass-aragonite is oxidizing ammonia. That is old news but points out that sand or maybe any rough surface can quickly be recolonized with ammonia oxidizing bacteria. Good news for “rip-cleaners”, right @brandon429 ? The point of interest is those AOB seem to be getting into the water either individually or as pieces of biofilm and rendering the water a weak ammonia oxidizer. Will that explanation work?So here's the results to this
ammonia added to one of the cycled tanks.
100mL of water-only in one sample
100mL of water with 2% (2mL) sand in the other sample.
samples on orbital shaker at 70rpm in the dark...
The sample with the newly cycled sand is processing at a rate of ~2ppm ammonia-nitrogen per day (about the same rate as the cycled tank the sample was pulled from).
The water only - less than 1/10 of that, ~0.15 ppm per day. It's probably not zero, but I can't really nail it down 100% because the water was filled with NO2/NO3 before I started, so I can be definitive and show the corresponding increase in NO2/NO3.)
I can say from this that no one should ever worry about losing nitrification capacity from doing a 90% water change after a completed cycle, you would lose an un-noticeable amount of nitrification.
(Unless you cycled with a product that isn't a chemoautotrophic nitrifier. Then all bets are off. Those heterotrophs could be anywhere.)
This also ties up the loose end for me: That the protocol I used (a few % sand in 100mL jar on an orbital shaker at 70rpm) is actually capable of detecting high nitrification rates of several ppm per day.
So those samples from my tank and the LFS that only nitrified a couple of tenths ppm per day, they simply didn't have many nitrifiers.
A single dose of biospira + worst possible live rock + dry sand + 9 day cycle = more than 10x the nitrification rate of my 10yr old sand bed.
- Joined
- Sep 21, 2018
- Messages
- 6,649
- Reaction score
- 7,136
I wonder whether the old sand bed has the amount of ammonia oxidizing capability it does because the organic amines and ammonia are being processed differently than in a new system. Said another way, the new aquarium starts out in an inorganic nutrient rich regime and moves to an organic nutrient rich one. Nitrification dominates early but then declines in importance and capability as the system ages and depends more on heterotrophic bacteria. The state of the nutrient regime might also have an influence on how biofilms mature and which nuisance photosynthesizers thrive in the system’s biofilm.So here's the results to this
ammonia added to one of the cycled tanks.
100mL of water-only in one sample
100mL of water with 2% (2mL) sand in the other sample.
samples on orbital shaker at 70rpm in the dark...

The sample with the newly cycled sand is processing at a rate of ~2ppm ammonia-nitrogen per day (about the same rate as the cycled tank the sample was pulled from).
The water only - less than 1/10 of that, ~0.15 ppm per day. It's probably not zero, but I can't really nail it down 100% because the water was filled with NO2/NO3 before I started, so I can be definitive and show the corresponding increase in NO2/NO3.)
I can say from this that no one should ever worry about losing nitrification capacity from doing a 90% water change after a completed cycle, you would lose an un-noticeable amount of nitrification.
(Unless you cycled with a product that isn't a chemoautotrophic nitrifier. Then all bets are off. Those heterotrophs could be anywhere.)
This also ties up the loose end for me: That the protocol I used (a few % sand in 100mL jar on an orbital shaker at 70rpm) is actually capable of detecting high nitrification rates of several ppm per day.
So those samples from my tank and the LFS that only nitrified a couple of tenths ppm per day, they simply didn't have many nitrifiers.
A single dose of biospira + worst possible live rock + dry sand + 9 day cycle = more than 10x the nitrification rate of my 10yr old sand bed.
Could a system with very strong ammonia oxidation capability be driving nuisance organism growth? Or is it the other way round? Or is the too rapid change from inorganic to organic regime causing dinoflagellates to dominate the biofilm? So many possibilities to look into.
- Joined
- May 22, 2016
- Messages
- 6,526
- Reaction score
- 10,058
That's pretty nice that you were able to catch AOB colonizing aragonite from the water in just a few days when they were unable to colonize glass in the same conditions.A point of interest. The biofilm that forms on glass slides in my system after 15 days do not oxidize ammonia but coat glass slides with aragonite and in five days the glass-aragonite is oxidizing ammonia. That is old news but points out that sand or maybe any rough surface can quickly be recolonized with ammonia oxidizing bacteria. Good news for “rip-cleaners”, right @brandon429 ? The point of interest is those AOB seem to be getting into the water either individually or as pieces of biofilm and rendering the water a weak ammonia oxidizer. Will that explanation work?
You are right that dry aragonite sand might be a best-case scenario for nitrifiers.
- Joined
- May 22, 2016
- Messages
- 6,526
- Reaction score
- 10,058
I'm not totally convinced that every system eventually settles to a low-substrate-nitrification equilibrium over time (Maybe just every system I get my hands on). Aquabiomics does find some systems that (at least as far as genetic sequences go) look like they possess a lot of nitrification. The BattleCorals system (article) was one of those.Said another way, the new aquarium starts out in an inorganic nutrient rich regime and moves to an organic nutrient rich one. Nitrification dominates early but then declines in importance and capability as the system ages and depends more on heterotrophic bacteria. The state of the nutrient regime might also have an influence on how biofilms mature and which nuisance photosynthesizers thrive in the system’s biofilm.
"Ammonia-oxidizing microbes made up almost 8% of your sample, which is higher than about 85% of aquariums I’ve tested, about twice as high as the average sample. Your sample also had high levels of nitrite-oxidizing bacteria (0.9%), which is among the highest of any tanks tested (higher than 96% of samples), and over 3-times higher than the average sample."
It's not a chemical test result, but a nearly 1% population of Nitrite Oxidizers is pretty strong evidence that a good amount of ammonia is not assimilated but is being processed through nitrification.
It's only one data point, but it's a tantalizing one, that a clearly desirable, coral-dominated system would have one of the highest measured nitrifying communities.
- Joined
- Sep 21, 2018
- Messages
- 6,649
- Reaction score
- 7,136
I agree. Unfortunately, we know virtually nothing about the systems Aquabiomics tests and finds or doesn’t finds nitrifyers. Hard to know if the information is important or a so what.I'm not totally convinced that every system eventually settles to a low-substrate-nitrification equilibrium over time (Maybe just every system I get my hands on). Aquabiomics does find some systems that (at least as far as genetic sequences go) look like they possess a lot of nitrification. The BattleCorals system (article) was one of those.
"Ammonia-oxidizing microbes made up almost 8% of your sample, which is higher than about 85% of aquariums I’ve tested, about twice as high as the average sample. Your sample also had high levels of nitrite-oxidizing bacteria (0.9%), which is among the highest of any tanks tested (higher than 96% of samples), and over 3-times higher than the average sample."
It's not a chemical test result, but a nearly 1% population of Nitrite Oxidizers is pretty strong evidence that a good amount of ammonia is not assimilated but is being processed through nitrification.
It's only one data point, but it's a tantalizing one, that a clearly desirable, coral-dominated system would have one of the highest measured nitrifying communities.
Also, we still don’t have a good characterization of what it means when we capture DNA in the water. Was it from an organism, cell debris or piece of biofilm? Does the predominant source of DNA vary from system to system and therefore the amount of nitrifyer found? We are in a similar situation as we are with ICP data: exciting, high tech stuff, but weak on validation of important parts of the procedure.
I think nitrification rate versus amount DNA collected would be one of several steps towards understanding what Aquabiomics data means.
Very interesting and exciting all the same.
- Joined
- Sep 1, 2019
- Messages
- 428
- Reaction score
- 330
I am not an academic but kind of feel like this might be what it’s like to hone in on a dissertation topic.
Anyhow, if only R2R awarded PhD’s.
This is fantastic reading. Thank you @taricha for doing actual research and pushing on questions that are central to how we think about N in reef aquariums. This gives me a lot to think about in managing my own box of saltwater.
Anyhow, if only R2R awarded PhD’s.
This is fantastic reading. Thank you @taricha for doing actual research and pushing on questions that are central to how we think about N in reef aquariums. This gives me a lot to think about in managing my own box of saltwater.
- Joined
- Jan 19, 2020
- Messages
- 1,380
- Reaction score
- 1,844
Neat experiment! Curious why the bio Spira was added? While low quality, the rock was live. Any testing done for nitrate?Each tank got Instant Ocean water, 500mL Caribsea aragonite dry sand, and low quality "live" rock from Petco. (The rocks had all been kept in a pile in the same tank. I imagine they are pretty similar). Each tank got the minimum recommended dose of Biospira, and were left to circulate with the lights off.
With regards to your system. Have you added any livestock recently? Or anything at all that could of brought in some goodies!I'm not totally convinced that every system eventually settles to a low-substrate-nitrification equilibrium over time
- Joined
- Sep 21, 2018
- Messages
- 6,649
- Reaction score
- 7,136
I am not an academic but kind of feel like this might be what it’s like to hone in on a dissertation topic.
Anyhow, if only R2R awarded PhD’s.
This is fantastic reading. Thank you @taricha for doing actual research and pushing on questions that are central to how we think about N in reef aquariums. This gives me a lot to think about in managing my own box of saltwater.
@taricha is always asking questions, always wondering how things work and then designing a clever experiment to answer the question. It is the scientific process in action.
- Joined
- May 22, 2016
- Messages
- 6,526
- Reaction score
- 10,058
Two reasons.Neat experiment! Curious why the bio Spira was added? While low quality, the rock was live. Any testing done for nitrate?
One, I wanted to replicate a low effort typical starting condition that many hobby tanks might experience.
Two, in reading through an aquabiomics article I was a little taken aback at how slow the nitrification response was from "maricultured live rock". I'll dig up this data later, but it leads me to believe the majority of the activity I saw in the first two weeks is from the Biospira bottle and maybe not the rock.
Not sure what livestock you are thinking that could push the nitrifier population way down.With regards to your system. Have you added any livestock recently? Or anything at all that could of brought in some goodies!
Algae and coral as consumers of ammonia seems to me a plenty sufficient explanation for the low nitrification activity of substrate.
- Joined
- Sep 21, 2018
- Messages
- 6,649
- Reaction score
- 7,136
Good point about activity from Biospira and not much from rock. If the typical live rock is like your “lazy sand”, the hobby might be overestimating the capacity of live rock to oxidize ammonia though it would be a excellent source of bacteria as Aquabiomics has demonstrated.Two reasons.
One, I wanted to replicate a low effort typical starting condition that many hobby tanks might experience.
Two, in reading through an aquabiomics article I was a little taken aback at how slow the nitrification response was from "maricultured live rock". I'll dig up this data later, but it leads me to believe the majority of the activity I saw in the first two weeks is from the Biospira bottle and maybe not the rock.
Not sure what livestock you are thinking that could push the nitrifier population way down.
Algae and coral as consumers of ammonia seems to me a plenty sufficient explanation for the low nitrification activity of substrate.
It would be interesting to know the ammonia oxidation rate for your live rock before adding anything. There might be a simple way to roughly estimate the volume and surface area so we could compare its capacity to your sand.
Two reasons.
One, I wanted to replicate a low effort typical starting condition that many hobby tanks might experience.
Two, in reading through an aquabiomics article I was a little taken aback at how slow the nitrification response was from "maricultured live rock". I'll dig up this data later, but it leads me to believe the majority of the activity I saw in the first two weeks is from the Biospira bottle and maybe not the rock.
If you haven't seen this already, you may find this interesting:
https://reefs.com/2017/12/17/new-study-live-rock-doesnt-really-denitrify/
https://www.researchgate.net/public...dissolved_inorganic_nitrogen_in_coral_aquaria
Similar threads
- Replies
- 38
- Views
- 676
- Replies
- 1
- Views
- 76
- Replies
- 12
- Views
- 311
- Replies
- 4
- Views
- 160



















