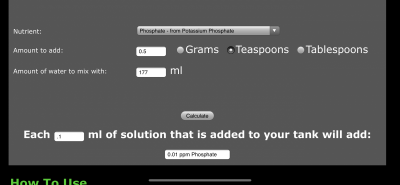
Greetings!
I have just started dosing phosphate using Seachem Flourish Phosphorus, but I figured that I should follow Randy's suggestion and make my own stock solution.
Can you please take a look at the following and advice on whether I should get Mono Sodium Phosphate Anhydrous or Sodium Phosphate Dibasic?

 apcpure.com
apcpure.com
Many thanks!
Cheers, Wilson
I have just started dosing phosphate using Seachem Flourish Phosphorus, but I figured that I should follow Randy's suggestion and make my own stock solution.
Can you please take a look at the following and advice on whether I should get Mono Sodium Phosphate Anhydrous or Sodium Phosphate Dibasic?

Sodium Phosphate Dibasic | Chemicals | APC Pure
APC Pure supplies Sodium Phosphate Dibasic ACS Reagent in all pack sizes for all applications
Many thanks!
Cheers, Wilson