I need a sanity check on my thinking here about how zinc from RO/DI source water would increase in my main system from evaporation alone without considering water changes.
If my RO/DI water is outputting water with zinc at 1ug/L and I have a 2100 gallon aquarium system that evaporates at most 50gallons/week or ~2600gallons/year then the zinc levels in the aquarium system would increase by approximately 1.24ug/L in the aquarium system due to evaporation.
As part of my investigation into determining the source of my high zinc levels I had my RO/DI water tested which came back at 1ug/L. While zinc is present in the source water I do not believe it is possible alone that this can be the cause of my high zinc issues as I don't believe based on the thinking above evaporation alone could create the concentrations I have in the system.
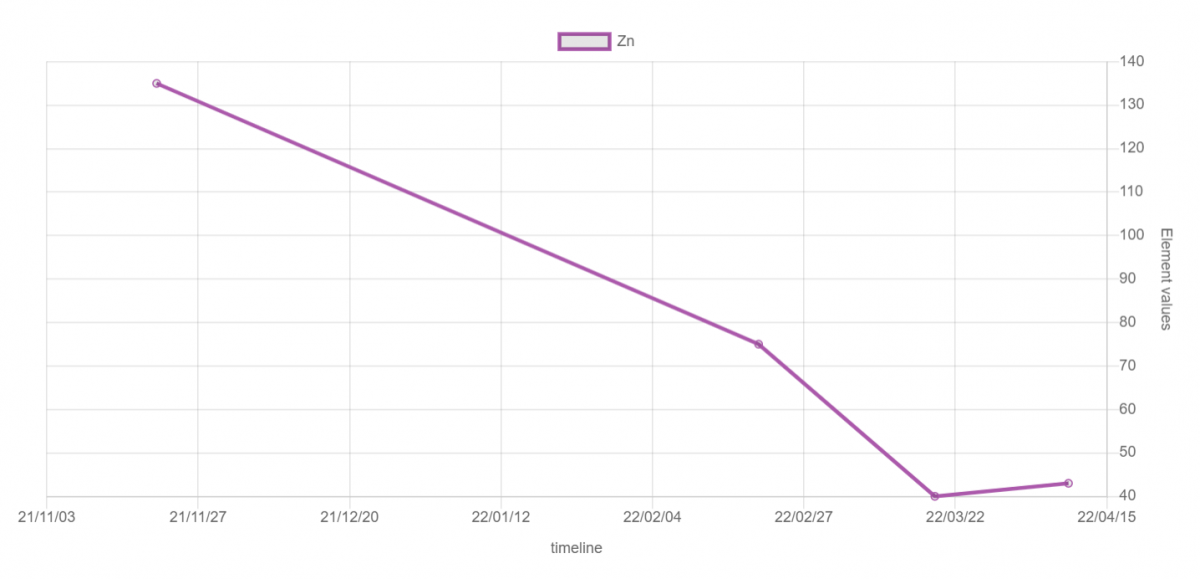
Zinc level history in aquarium: I have been doing a series of water changes which lowered the zinc levels. I found some corroding magnets that might have been zinc coated but they were removed on March 1st just before a large water change of 500gallons and a round a metal absorbers which got zinc down to 40ug/L. I did another round of metal absorbers and a week after they were in I sent off another ICP test which came back with 43ug/L. This is when I decided to send in source RO/DI water which came back at 1ug/L. I believe I still have an active source of zinc as I don't believe the evaporation could cause 3ug/L increase system wide during a week where I had used detox for three days followed by a week of: 2xmetasorbs, 3 bags of fresh carbon, and 6 poly filters. If anyone can confirm my thinking on how evaporation would increase zinc above it would be much appreciated. I am going to proceed with another water change and remove all the travertine tiles from my system as they might be a potential source of zinc and it is the fist time I have ever used them in a system before. I also have to inspect my threaded rod cross braces as they are galvanized with zinc but they are incased in PVC that is sealed with silicone and not directly exposed to any saltwater over the tanks.
If my RO/DI water is outputting water with zinc at 1ug/L and I have a 2100 gallon aquarium system that evaporates at most 50gallons/week or ~2600gallons/year then the zinc levels in the aquarium system would increase by approximately 1.24ug/L in the aquarium system due to evaporation.
As part of my investigation into determining the source of my high zinc levels I had my RO/DI water tested which came back at 1ug/L. While zinc is present in the source water I do not believe it is possible alone that this can be the cause of my high zinc issues as I don't believe based on the thinking above evaporation alone could create the concentrations I have in the system.
Zinc level history in aquarium: I have been doing a series of water changes which lowered the zinc levels. I found some corroding magnets that might have been zinc coated but they were removed on March 1st just before a large water change of 500gallons and a round a metal absorbers which got zinc down to 40ug/L. I did another round of metal absorbers and a week after they were in I sent off another ICP test which came back with 43ug/L. This is when I decided to send in source RO/DI water which came back at 1ug/L. I believe I still have an active source of zinc as I don't believe the evaporation could cause 3ug/L increase system wide during a week where I had used detox for three days followed by a week of: 2xmetasorbs, 3 bags of fresh carbon, and 6 poly filters. If anyone can confirm my thinking on how evaporation would increase zinc above it would be much appreciated. I am going to proceed with another water change and remove all the travertine tiles from my system as they might be a potential source of zinc and it is the fist time I have ever used them in a system before. I also have to inspect my threaded rod cross braces as they are galvanized with zinc but they are incased in PVC that is sealed with silicone and not directly exposed to any saltwater over the tanks.















