Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Has anyone successfully raised Sunshine Chromis from fry?
- Thread starter RSNJReef
- Start date
- Tagged users None
Hey Nano, yeah, it seems like with every batch the survival rate is improving. I’m not going to pretend like I know myself about effectively raising fry, but this a fun yet tedious experience (takes about a half hour every morning and about 45 min every afternoon with tending to the fry system, the rotifer culture, the apocyclops culture, and the parvo culture). But I’m finding this to be interesting and fun, learning a lot of stuff here so definitely want to keep going until I completely fail or I managed to get the fry through metamorphosis, then will see where it goes from there.
either way, thanks guys for the support and help, it’s keeping me going through this and I really appreciate it!!
either way, thanks guys for the support and help, it’s keeping me going through this and I really appreciate it!!
Ok, another post for tonight. The fry are still mostly there, though it looks like the numbers dropped in the two fish bowl was from 50/30 to around 40/25 or so.
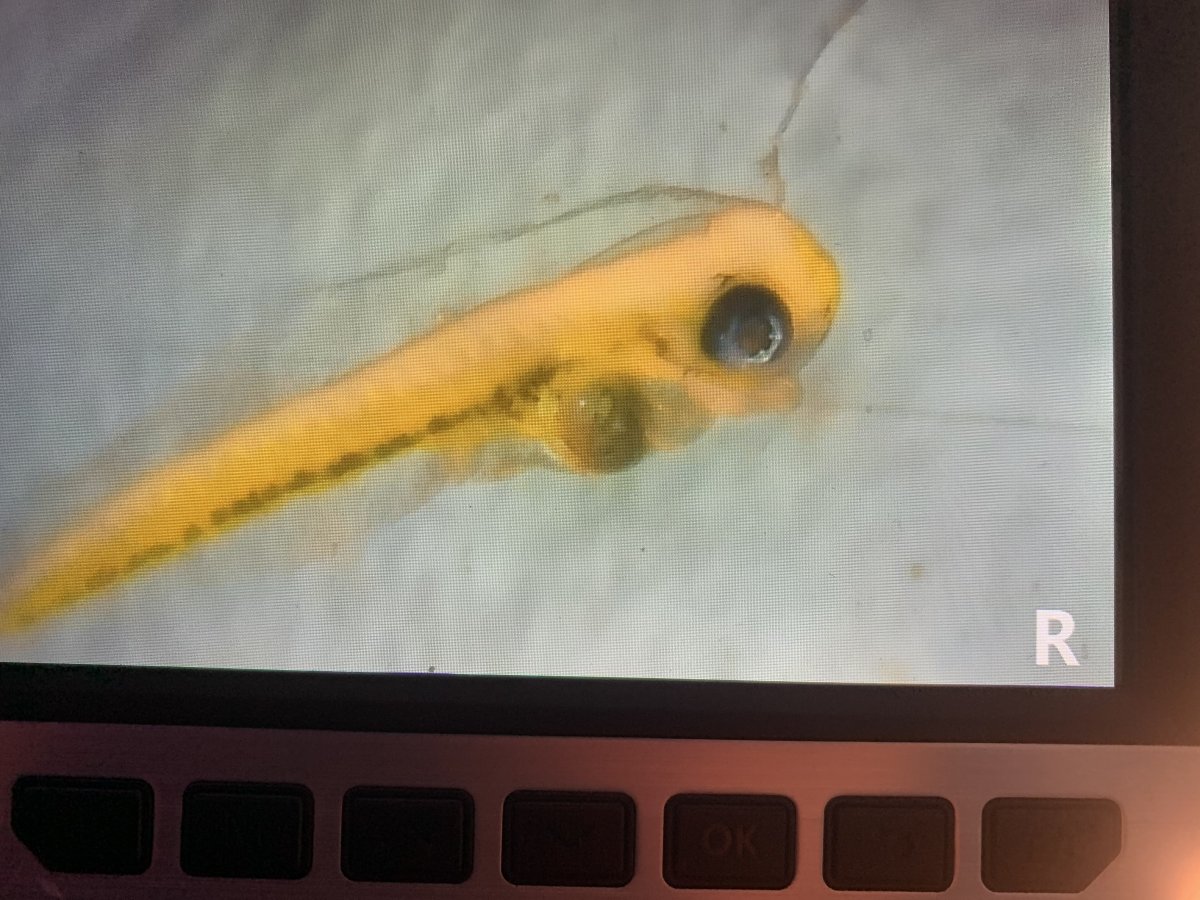
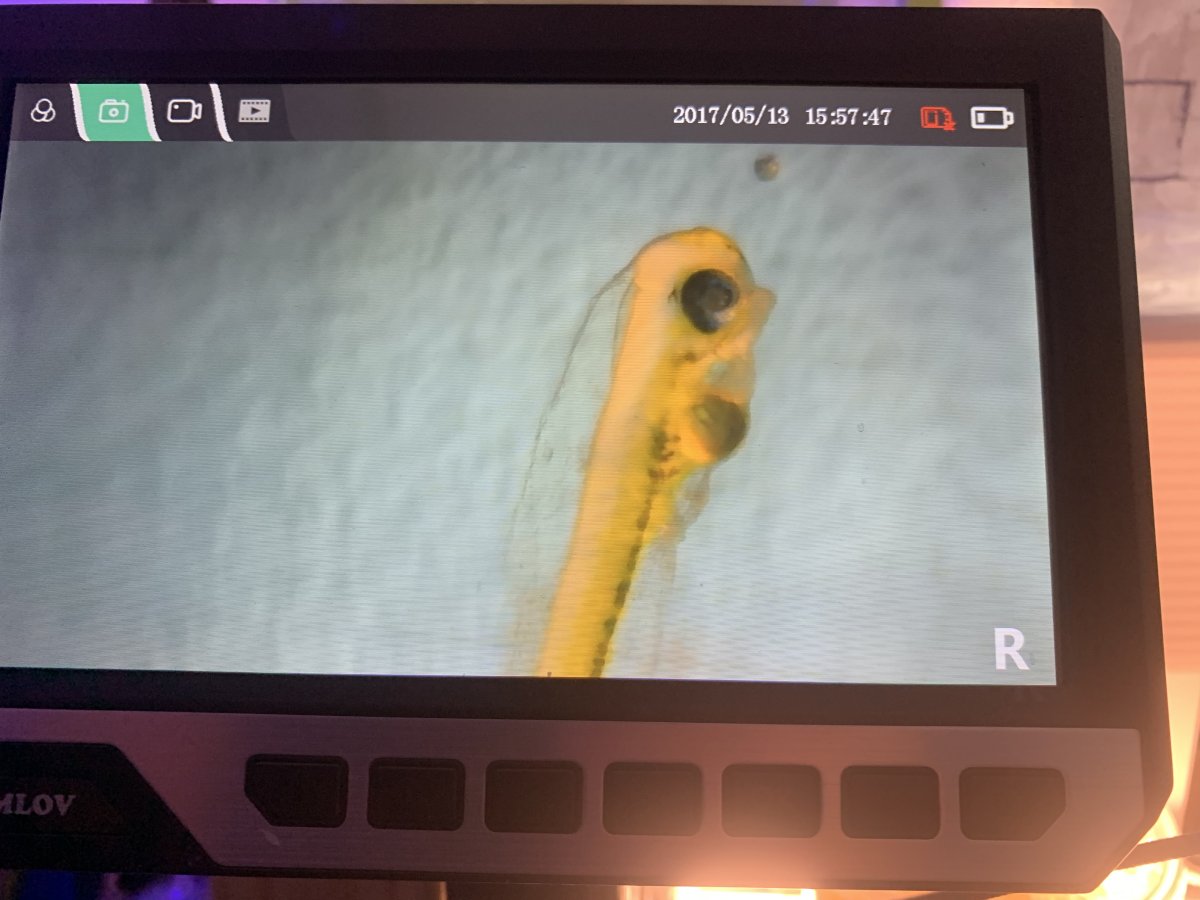
the fry are at different developmental stages, I collected 3 of them and the first looked like it still had a little of its yolk sac left but the stomach and intestines were forming. One of the others seemed to have used up all of its yolk sac and a formed stomach, and, I’m not sure if this one was eating or not because I couldn’t see any physical copepods in their system, but their stomach looked swollen and brown , which might be an indication of digested copepods, not sure. I’m hopeful they are eating, but I guess I’ll know for sure within the next 24-48 hours.
pics below of two of the fry, the first is the one with remnants of its yolk sac, and the other with the brown stomach.
Fry 1


Fry 2
the fry are at different developmental stages, I collected 3 of them and the first looked like it still had a little of its yolk sac left but the stomach and intestines were forming. One of the others seemed to have used up all of its yolk sac and a formed stomach, and, I’m not sure if this one was eating or not because I couldn’t see any physical copepods in their system, but their stomach looked swollen and brown , which might be an indication of digested copepods, not sure. I’m hopeful they are eating, but I guess I’ll know for sure within the next 24-48 hours.
pics below of two of the fry, the first is the one with remnants of its yolk sac, and the other with the brown stomach.
Fry 1
Fry 2
Last edited:
You are doing great work. Following along.
Thanks Hyperman. Hoping that the parvo will work for these little guys, but, we’ll see soon. If nothing else, I’m definitely learning a lot here.You are doing great work. Following along.
Thanks Hyperman. Hoping that the parvo will work for these little guys, but, we’ll see soon. If nothing else, I’m definitely learning a lot here.
You've taken some really dope initiative with all of these cultures. Inspiring me to start running some fishbowls here.
I say go for it man, just remember it’s a little bit of work (once you got your system down it can be a half hour every morning and 45 min or an hour every afternoon if you want to keep multiple cultures going). It is interesting and fun doing this though. I’m no breeder, but if you got any questions I can try to help.You've taken some really dope initiative with all of these cultures. Inspiring me to start running some fishbowls here.
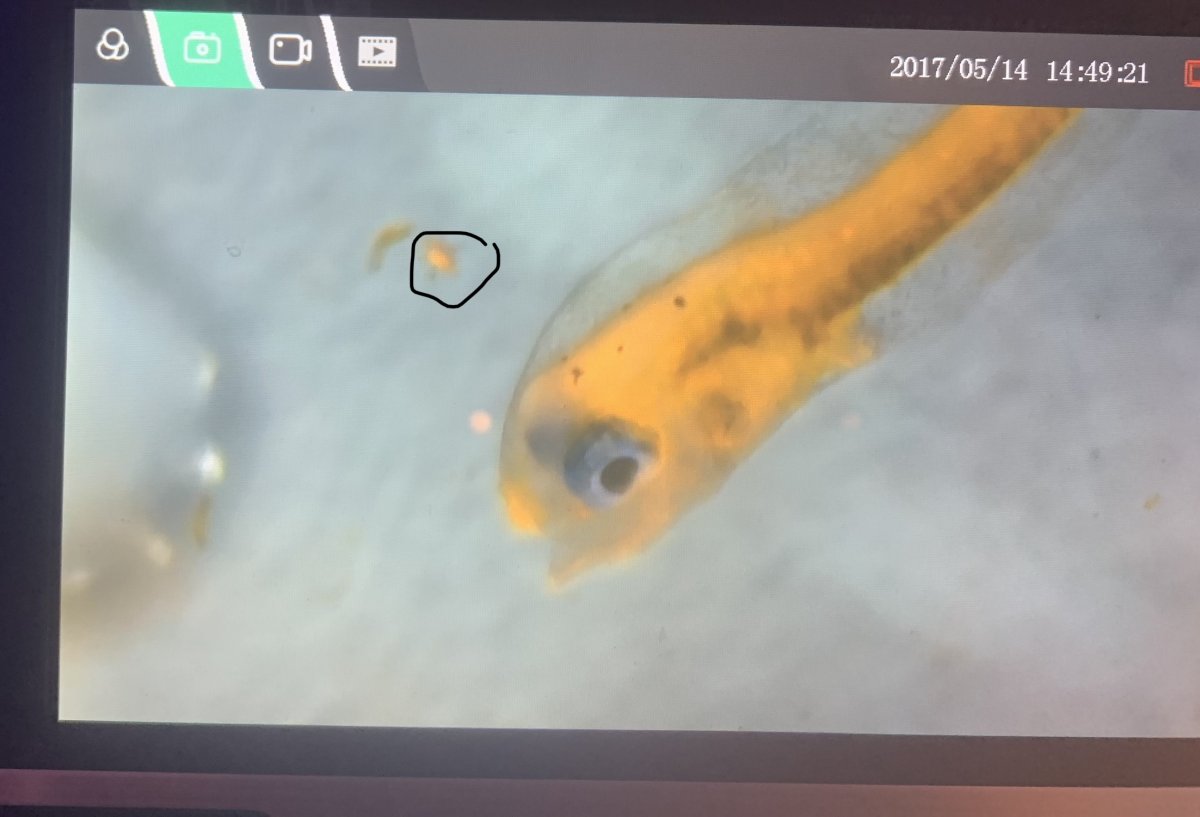
Ok, another update. I lost quite a few of the fry, as of an hour ago the original 50/30 is now down to about 15/7. I had been feeding the parvo nauplii every morning and afternoon for the last couple of days, and, the density of the parvo looks pretty decent constantly (is about 2-3 per ml), but I’m noticing that the density of the copepods isn’t really going down much after 12 hours. In looking at the gut of the fry, I also don’t see any anything that looks like the parvo in their stomach, though, I got a picture of the size of the parvo next to one of the fry and I don’t think it’s the size of the nauplii that’s a problem, it looks like they can easily fit into the mouth of the fry.
something interesting I have saw this evening though. So I’ve set the aeration in the fishbowls up so that the rolling motion is a gentle roll, so the copepods would constantly be in a gentle slow roll through the tank around the fry. At first when I got to the fishbowls, I thought majority of the fry had died and there were only 4-5 left in each fish bowl. I wanted to try and collect one of the dead fry to examine it, so looked closely at the bottom of the fish bowl. What I saw was around 5-10 fish settled to the bottom of the fishbowl, and they were just sitting there, and would move around along the bottom every 3-4 seconds, like they were making small dashes along the bottom of the tank. I used a pipette to agitate the water a bit, and the fry at the bottom of the tank then stirred up and went back into the water column as they had been earlier today. After seeing this I turned up the airlines a little to make the rolling flow of the fishbowls a little faster, but not to the point that I felt the fry would go crashingg go into things or each other.
I think tomorrow will be the decisive day as to whether the current method worked or not. I don’t think it’s the food size that’s an issue, the only think I can think of is that the density wasn’t right, or that the motion of the parvo is not enticing to the fry.
the breeding pair of chromis just laid another batch of eggs. I sat and watched the entire thing (well, for the first part of the egg batch at least), and, it was interesting. The male and female went for 20 minutes laying eggs and fertilizing them, then, for the next ten minutes after that, the male would pass over the eggs again every 1-2 minutes fluttering and trying to fertilize them. Then, after those 10 minutes were up, the female came in again and laid eggs again for about 10 minutes while the male passed after to fertilize them. Now it makes sense how the egg batch seemed to have different growth rates, it’s cause the eggs were laid at different times through the day I believe.
I’ll collect more eggs and add them to the system tomorrow, what I may do is collect all of the older fry from the last 1-2 days and move them to one of the fishbowls, then move the new eggs to the other fishbowl. I may also start trying to feed a little bit of b12 to the water to see if it would help the appetite. I’m also going to try and feed a bit more phytoplankton to the tank to make it a little more cloudy, as I’ve been feeding the phyto pretty sparingly and the fish bowls have been pretty clear except for the parvo and the fry.
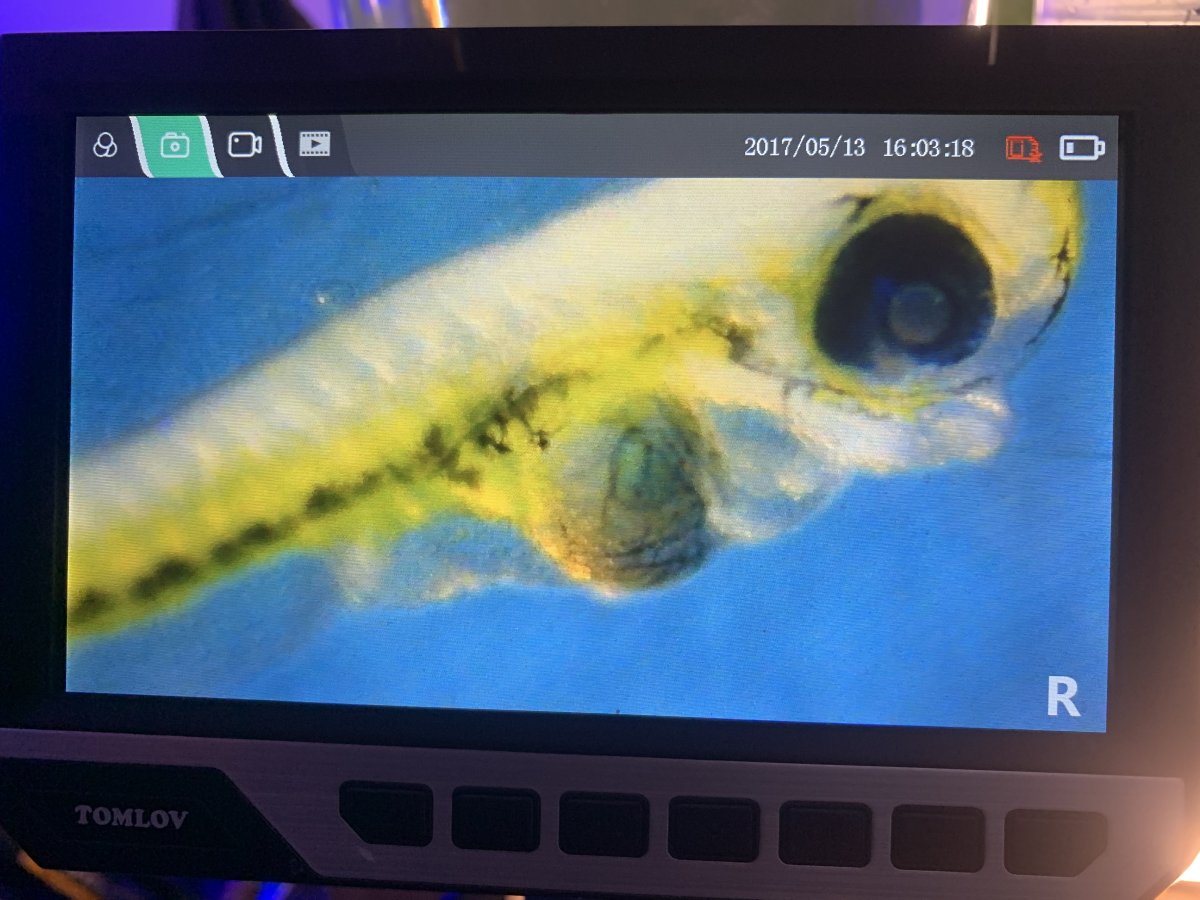
here’s pi’s of on of the fry, you can see in some of the pics the stomach and intestines look to be formed, and it also has developed its pelvic fins). Also in two of the pics below you’ll see two black circles, which are of a parvo nauplii (in one pic) and a parvo egg (in the other pic) for a size comparison to the fry’s mouth.




fry # 2



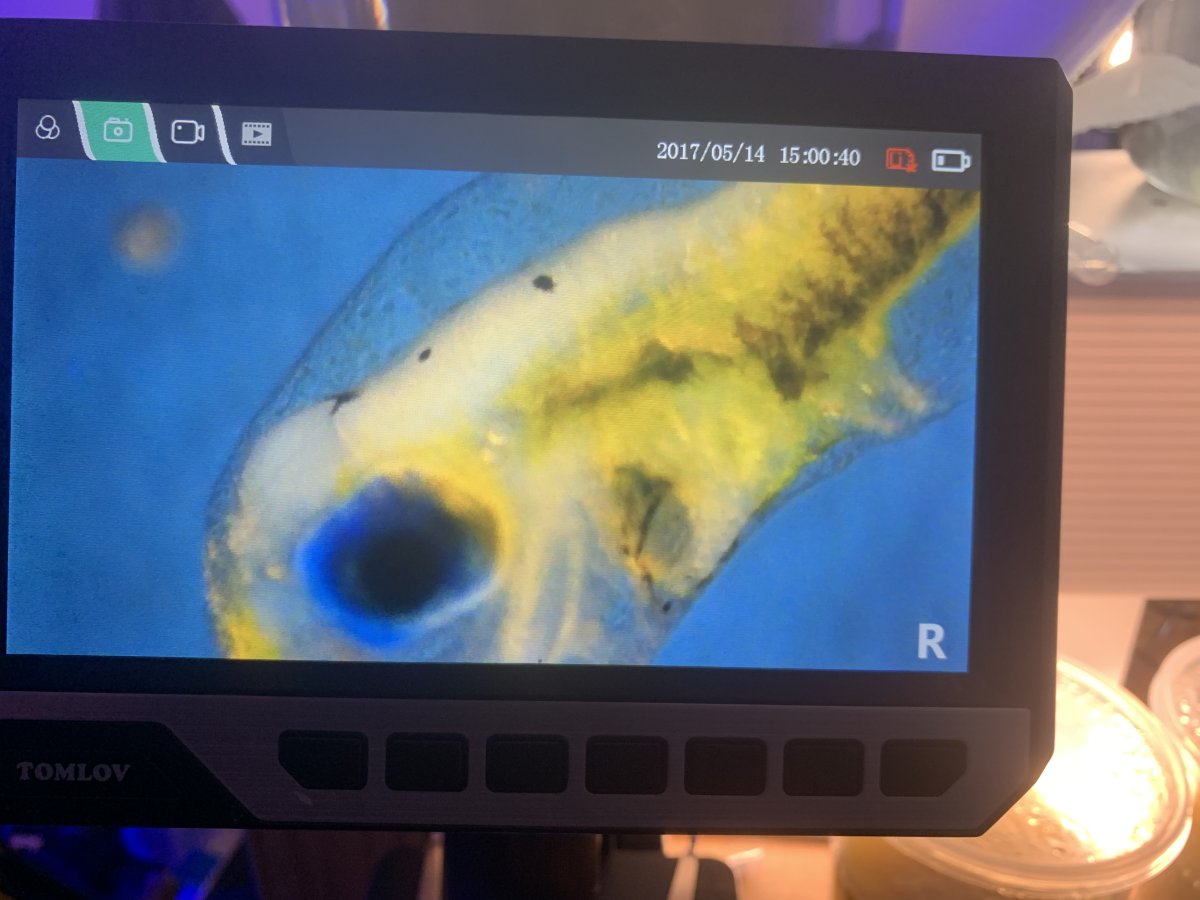


something interesting I have saw this evening though. So I’ve set the aeration in the fishbowls up so that the rolling motion is a gentle roll, so the copepods would constantly be in a gentle slow roll through the tank around the fry. At first when I got to the fishbowls, I thought majority of the fry had died and there were only 4-5 left in each fish bowl. I wanted to try and collect one of the dead fry to examine it, so looked closely at the bottom of the fish bowl. What I saw was around 5-10 fish settled to the bottom of the fishbowl, and they were just sitting there, and would move around along the bottom every 3-4 seconds, like they were making small dashes along the bottom of the tank. I used a pipette to agitate the water a bit, and the fry at the bottom of the tank then stirred up and went back into the water column as they had been earlier today. After seeing this I turned up the airlines a little to make the rolling flow of the fishbowls a little faster, but not to the point that I felt the fry would go crashingg go into things or each other.
I think tomorrow will be the decisive day as to whether the current method worked or not. I don’t think it’s the food size that’s an issue, the only think I can think of is that the density wasn’t right, or that the motion of the parvo is not enticing to the fry.
the breeding pair of chromis just laid another batch of eggs. I sat and watched the entire thing (well, for the first part of the egg batch at least), and, it was interesting. The male and female went for 20 minutes laying eggs and fertilizing them, then, for the next ten minutes after that, the male would pass over the eggs again every 1-2 minutes fluttering and trying to fertilize them. Then, after those 10 minutes were up, the female came in again and laid eggs again for about 10 minutes while the male passed after to fertilize them. Now it makes sense how the egg batch seemed to have different growth rates, it’s cause the eggs were laid at different times through the day I believe.
I’ll collect more eggs and add them to the system tomorrow, what I may do is collect all of the older fry from the last 1-2 days and move them to one of the fishbowls, then move the new eggs to the other fishbowl. I may also start trying to feed a little bit of b12 to the water to see if it would help the appetite. I’m also going to try and feed a bit more phytoplankton to the tank to make it a little more cloudy, as I’ve been feeding the phyto pretty sparingly and the fish bowls have been pretty clear except for the parvo and the fry.
here’s pi’s of on of the fry, you can see in some of the pics the stomach and intestines look to be formed, and it also has developed its pelvic fins). Also in two of the pics below you’ll see two black circles, which are of a parvo nauplii (in one pic) and a parvo egg (in the other pic) for a size comparison to the fry’s mouth.
fry # 2

Last edited:
If the food's still not right, then you can try Oithona colcarva nauplii (you would need to either reach out Algaebarn and see if they'd be willing to sell you a starter culture so that you can try to use them in rearing these guys, or order some of their galaxy pods and try to hand filter the Oithona into their own container - this would take forever and be really difficult, so it's not a course that I would take unless absolutely necessary).I think tomorrow will be the decisive day as to whether the current method worked or not. I don’t think it’s the food size that’s an issue, the only think I can think of is that the density wasn’t right, or that the motion of the parvo is not enticing to the fry.
From the green chromis rearing by Rising Tide (link on page 1 - this is why I'd suggest Oithona, which have a reported nauplii size of 45 microns):
"Two days after hatching, the larvae are ready to feed, and must be supplied with tiny copepod nauplii to survive. Chromis larvae, in our experience, are highly selective in choosing their zooplankton prey. We offered cultured Oithona colcarva nauplii at a density of about 3/mL. The first feeding stage of marine fish larvae is typically characterized as the stage prone to the highest levels of mortality. We experienced little early mortality since the larvae fed aggressively on the cultured Oithona. Bottlenecks to culture developed after day 10 and 15 when the larvae required sequentially larger copepod prey. Flexion occurred near day 15, which was accompanied by our highest mortality rates. At this stage of development larvae undergo dramatic changes in morphology and reorganization of the gut. We suspect that mortality here is attributed to insufficient nutrition through early development."
This is a really good find ISFTS, thank you for this. Oithona is one of those species I had been looking at from afar, but never tried my hand at it cause one it was nearly impossible to find a pure culture or it anywhere, and at the time there wasn’t a ton of info on how to sustain the culture long term. I may try this in one of the next future rounds, but want to try another go with the parvo one more time, but with a vitamin supplement routine in this one.If the food's still not right, then you can try Oithona colcarva nauplii (you would need to either reach out Algaebarn and see if they'd be willing to sell you a starter culture so that you can try to use them in rearing these guys, or order some of their galaxy pods and try to hand filter the Oithona into their own container - this would take forever and be really difficult, so it's not a course that I would take unless absolutely necessary).
From the green chromis rearing by Rising Tide (link on page 1 - this is why I'd suggest Oithona, which have a reported nauplii size of 45 microns):
"Two days after hatching, the larvae are ready to feed, and must be supplied with tiny copepod nauplii to survive. Chromis larvae, in our experience, are highly selective in choosing their zooplankton prey. We offered cultured Oithona colcarva nauplii at a density of about 3/mL. The first feeding stage of marine fish larvae is typically characterized as the stage prone to the highest levels of mortality. We experienced little early mortality since the larvae fed aggressively on the cultured Oithona. Bottlenecks to culture developed after day 10 and 15 when the larvae required sequentially larger copepod prey. Flexion occurred near day 15, which was accompanied by our highest mortality rates. At this stage of development larvae undergo dramatic changes in morphology and reorganization of the gut. We suspect that mortality here is attributed to insufficient nutrition through early development."
mom going to harvest the next batch of eggs tonight and try the following
clean the larval culture system out and get rid of all remaining parvo and dead fry (I was only able to see one fry this morning during feeding, and don’t think that one will survive to tonight).
collect the eggs and give them a one hour bath in 100 ppm hydrogen peroxide solution.
Lower the salinity of the fry system to be 1.020-1.022 so it’s easier on their system.
Feed the culture system a stock solution of vitachem and vitamin b12 added twice a day.
Also add the probiotic sanolife mic-f to the culture system daily to help with gut health.
muse purely tetraselmis phytoplankton within the fry tank to cloud the water (was using isochrysis but now going to use a motile species of phyto on the chance the fry take a liking the movement of the tetraselmis better.
do 50% water changes to reduce any fouling of the water from the vitamins.
Going to try all of this on the next round and see where it goes. Want to try my hand at what would be available to the regular consumer before I start getting into specialized feeds like the Oithona (right now trying methods to anyone can do if they have the time and patience). If all of this fails though, then will try the infusoria and if that doesn’t work, then will try to see if I can source the Oithona.
Through this though ISFTS, the knowledge you’re throwing out there is invaluable. With all of this knowledge, you’d make a great breeder (how come you’re not breeding too?). Thanks for all of this.
Thank you - I don't have a tank right now (my landlords won't allow it) or I would be.With all of this knowledge, you’d make a great breeder (how come you’re not breeding too?).
Thanks drawman!!! I can say I waited a long time for a moment like this (a breeding pair of fish), and gonna try everything I can to get this right. Definitely a labor of love, but I’m having fun doing this. Hopefully will get there in time.Following amazing writeup and great progress so far!
Ok, another update for tonight. I had expected thatxx CS all of the fry would be dead by tonight, but, surprisingly enough, two had survived. I collected the remaining two and looked at them under the microscope, and, to my surprise, one of them was pooping. See ictures below:



This wasxx CS a promising sign that they were eating something, and, gives me some hope that the parvocalanus would work, but there’s something missing from their diet.
as I mentioned earlier today, my plan with the next batch of eggs is to collect and wash them in hydrogen peroxide. So I collected another batch of eggs today and now it looks like the male recognizes the siphon tube. The second I put it into the tank he immediately began attacking it, so I collected about a third of the eggs and left the other two thirds behind. I’m noticing that the egg clusters that the fish are laying are getting bigger each time. This cluster looked to be a good 500 eggs or so, so I collected around 100-200 eggs. I put the eggs into a solution of 30 ounces tank water and 50ml of 3-% peroxide solution. I let the eggs sit in this solution for an hour then added them to the fry system (I cleaned out the system, rinsed everything out really well, and then added fresh clean saltwater (not tank water this time) with a salinity of 1.020 specific gravity.
I collected a couple of the eggs and looked at them under the microscope, and I think I’m going to use the peroxide solution for washing the eggs from now on, these eggs looked way cleaner than any of the previous eggs I had collected. See below:

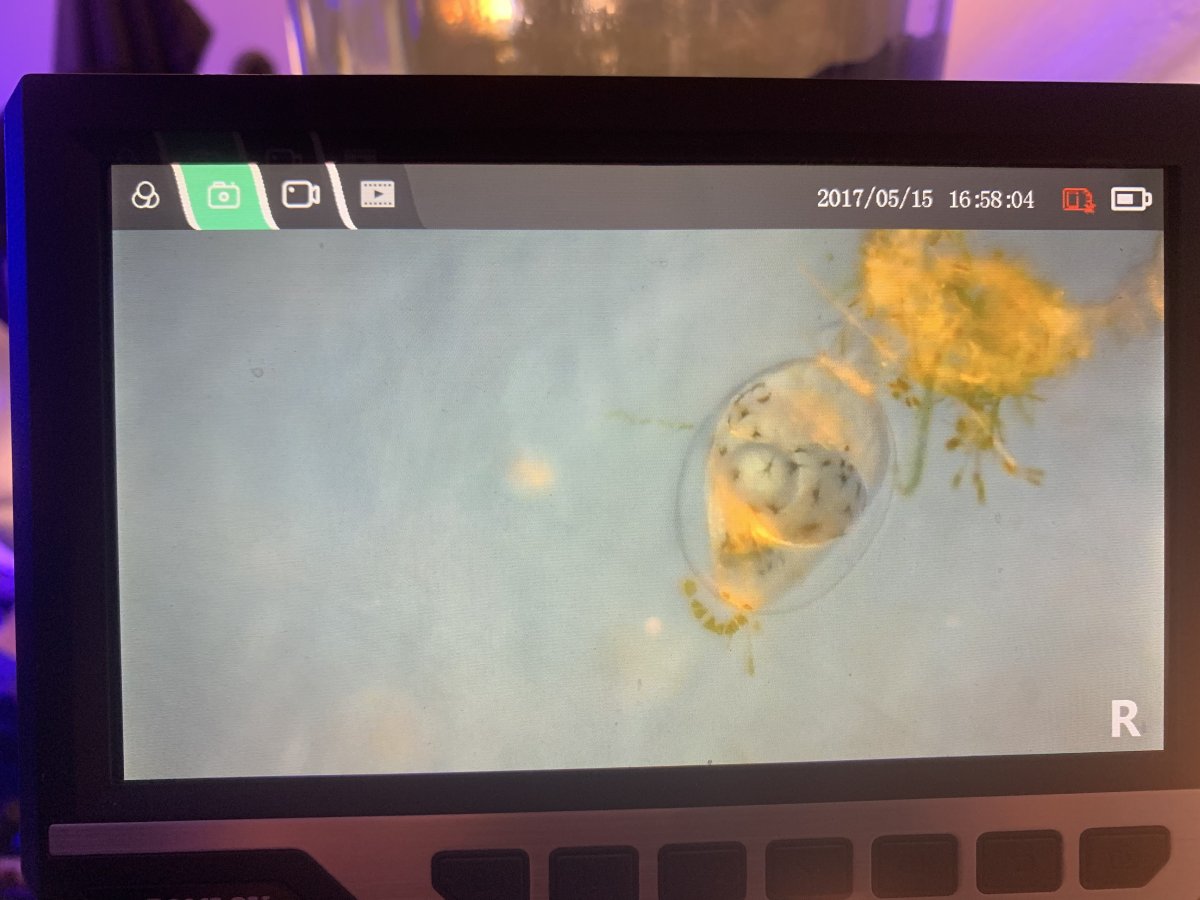
I was also surprised as to far developed these eggs were. From the last clusters, the development time seemed to be longer, and, the way the eggs looked and the fact that the eggs were twitching already, the embryo in the egg would twitch or roll in the egg every 5 seconds or so. I’m guessing based on what I saw tonight that the eggs will hatch starting tomorrow night. Starting tomorrow night I’ll start adding the sanolife mic-f and the vitachem/b12 stock mix in preparation for when they deplete their yolk sacs, and, will start doing 30% water changes daily to try and keep the water fouling down. Will see how this round goes, but it amazes me how every new batch I get from these fish seem to be even better than the last.
This wasxx CS a promising sign that they were eating something, and, gives me some hope that the parvocalanus would work, but there’s something missing from their diet.
as I mentioned earlier today, my plan with the next batch of eggs is to collect and wash them in hydrogen peroxide. So I collected another batch of eggs today and now it looks like the male recognizes the siphon tube. The second I put it into the tank he immediately began attacking it, so I collected about a third of the eggs and left the other two thirds behind. I’m noticing that the egg clusters that the fish are laying are getting bigger each time. This cluster looked to be a good 500 eggs or so, so I collected around 100-200 eggs. I put the eggs into a solution of 30 ounces tank water and 50ml of 3-% peroxide solution. I let the eggs sit in this solution for an hour then added them to the fry system (I cleaned out the system, rinsed everything out really well, and then added fresh clean saltwater (not tank water this time) with a salinity of 1.020 specific gravity.
I collected a couple of the eggs and looked at them under the microscope, and I think I’m going to use the peroxide solution for washing the eggs from now on, these eggs looked way cleaner than any of the previous eggs I had collected. See below:
I was also surprised as to far developed these eggs were. From the last clusters, the development time seemed to be longer, and, the way the eggs looked and the fact that the eggs were twitching already, the embryo in the egg would twitch or roll in the egg every 5 seconds or so. I’m guessing based on what I saw tonight that the eggs will hatch starting tomorrow night. Starting tomorrow night I’ll start adding the sanolife mic-f and the vitachem/b12 stock mix in preparation for when they deplete their yolk sacs, and, will start doing 30% water changes daily to try and keep the water fouling down. Will see how this round goes, but it amazes me how every new batch I get from these fish seem to be even better than the last.
Ok, another update for tonight. I had expected thatxx CS all of the fry would be dead by tonight, but, surprisingly enough, two had survived. I collected the remaining two and looked at them under the microscope, and, to my surprise, one of them was pooping. See ictures below:



This wasxx CS a promising sign that they were eating something, and, gives me some hope that the parvocalanus would work, but there’s something missing from their diet.
as I mentioned earlier today, my plan with the next batch of eggs is to collect and wash them in hydrogen peroxide. So I collected another batch of eggs today and now it looks like the male recognizes the siphon tube. The second I put it into the tank he immediately began attacking it, so I collected about a third of the eggs and left the other two thirds behind. I’m noticing that the egg clusters that the fish are laying are getting bigger each time. This cluster looked to be a good 500 eggs or so, so I collected around 100-200 eggs. I put the eggs into a solution of 30 ounces tank water and 50ml of 3-% peroxide solution. I let the eggs sit in this solution for an hour then added them to the fry system (I cleaned out the system, rinsed everything out really well, and then added fresh clean saltwater (not tank water this time) with a salinity of 1.020 specific gravity.
I collected a couple of the eggs and looked at them under the microscope, and I think I’m going to use the peroxide solution for washing the eggs from now on, these eggs looked way cleaner than any of the previous eggs I had collected. See below:


I was also surprised as to far developed these eggs were. From the last clusters, the development time seemed to be longer, and, the way the eggs looked and the fact that the eggs were twitching already, the embryo in the egg would twitch or roll in the egg every 5 seconds or so. I’m guessing based on what I saw tonight that the eggs will hatch starting tomorrow night. Starting tomorrow night I’ll start adding the sanolife mic-f and the vitachem/b12 stock mix in preparation for when they deplete their yolk sacs, and, will start doing 30% water changes daily to try and keep the water fouling down. Will see how this round goes, but it amazes me how every new batch I get from these fish seem to be even better than the last.
Awesome!
Just curious, how do you plan to handle the water changes?
I don't know if it is at all necessary, but my idea when we get marine eggs was to use an airline tube to syphon out with some fine foam on it to prevent sucking anything valuable up while also siphoning in at the same rate the fresh water to avoid changing the wayer too drastically wheb doing larger than a 10% change. Is this at all necessary?
Yeah, the third link I shared about breeding Azurina cyanea mentioned that it took them almost a year before they had fish start surviving (with seemingly no explanation, as I understand it, as they don’t mention making any changes to the methods they were using to try and rear the young):Will see how this round goes, but it amazes me how every new batch I get from these fish seem to be even better than the last.
“Over 11 months, the Blue Chromis broodstock laid multiple nests two to three times each month. Out of those nests, about 80% of them were removed for an attempt at rearing, and 100% of those rearing attempts were unsuccessful past 23 days post-hatch (dph). In May 2016, one particular rearing attempt started out like all of the other previous attempts: there was a fairly high rate of mortality within the first two weeks of growth, then around 15 dph, the larvae started to develop their long pelvic fins.”
Hey Hyperman, yeah, had accounted for that in the initial setup of these fish bowls. I’ll take some pics of the system later, but the two fish bowls were modified with an overflow that dumps into a 1 gallon jar. The jar has a fountain pump which feeds water continuously to each fish bowl, and the fishbowls them dump the overflow back into the jar. I also put fine mesh sponge on the inlet side of the overflow to keep the fry in the fishbowls and not the jar. I also set up the airlines (which cause the rolling motion of the water and fry) to be close to perpendicular to where the overflows are, so the rolling motion does not have the fry floating directly into the coarse sponge. So far it’s been working well and haven’t had any fry flying into the sponge, and when I do the water change I just change the water in the jar, which then feeds the fish bowls.Awesome!
Just curious, how do you plan to handle the water changes?
I don't know if it is at all necessary, but my idea when we get marine eggs was to use an airline tube to syphon out with some fine foam on it to prevent sucking anything valuable up while also siphoning in at the same rate the fresh water to avoid changing the wayer too drastically wheb doing larger than a 10% change. Is this at all necessary?
will take pics later to show the setup.
Last edited:
Yeah I read the link and it’s kind of crazy how long the development time of the brood stock takes, but I guess it makes sense in a way cause the brood stock is still trying to figure things out on their own.Yeah, the third link I shared about breeding Azurina cyanea mentioned that it took them almost a year before they had fish start surviving (with seemingly no explanation, as I understand it, as they don’t mention making any changes to the methods they were using to try and rear the young):
“Over 11 months, the Blue Chromis broodstock laid multiple nests two to three times each month. Out of those nests, about 80% of them were removed for an attempt at rearing, and 100% of those rearing attempts were unsuccessful past 23 days post-hatch (dph). In May 2016, one particular rearing attempt started out like all of the other previous attempts: there was a fairly high rate of mortality within the first two weeks of growth, then around 15 dph, the larvae started to develop their long pelvic fins.”
Love this write-up, will follow along
Similar threads
- Replies
- 8
- Views
- 294
- Replies
- 3
- Views
- 834



















