As the temperature outside gets colder, there are a number of threads being posted on heat packs with questions. I did this study several years ago but can't find it in the R2R search function, so I may not have posted it on this site, only locally.
Since this information is still relevant, it may be helpful to review and open it to discussion.
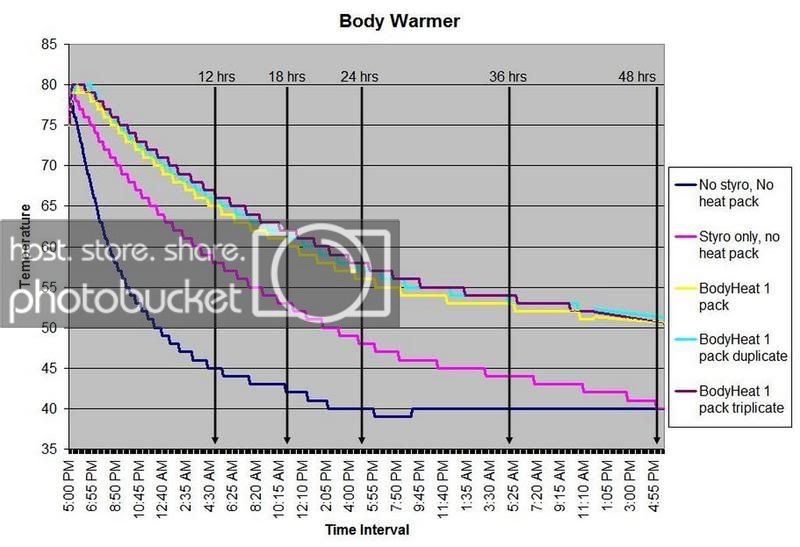
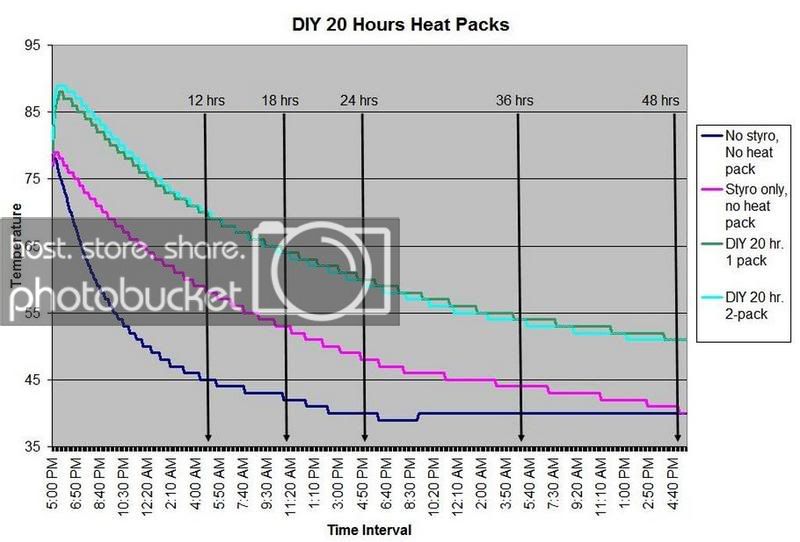
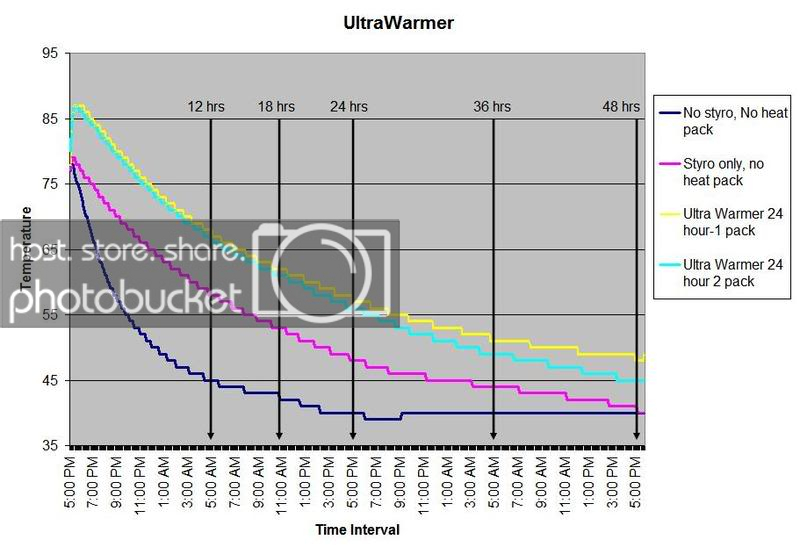
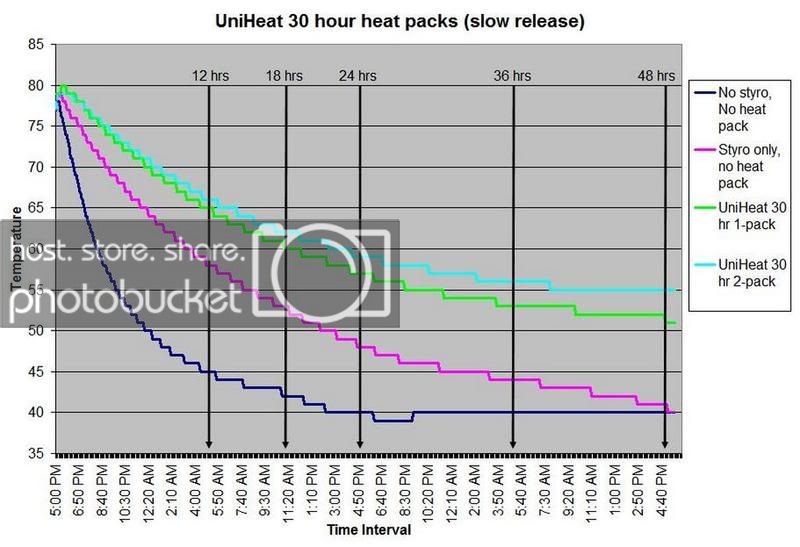
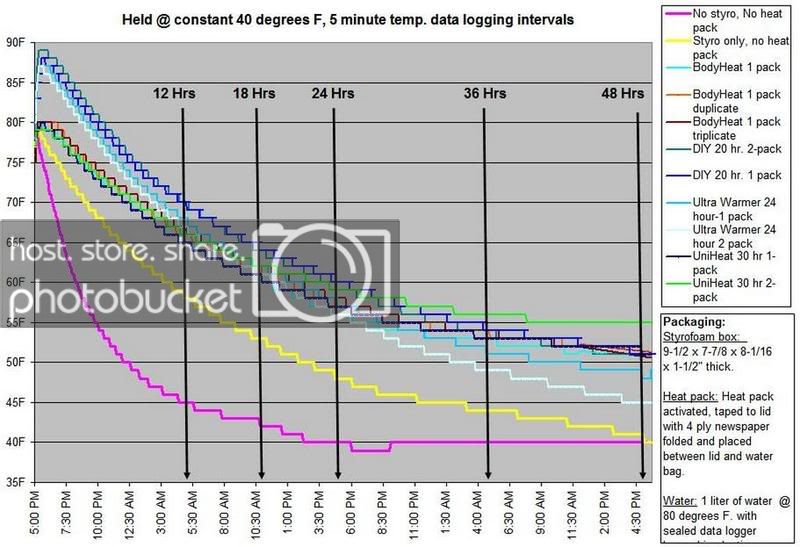
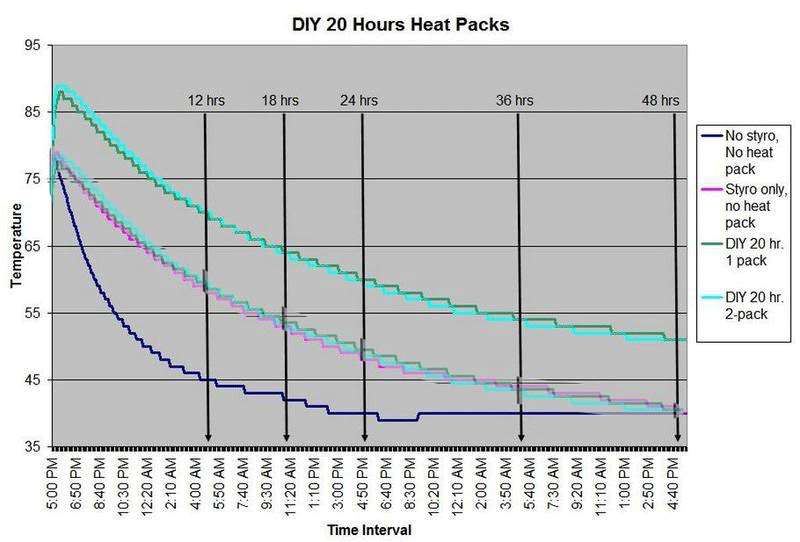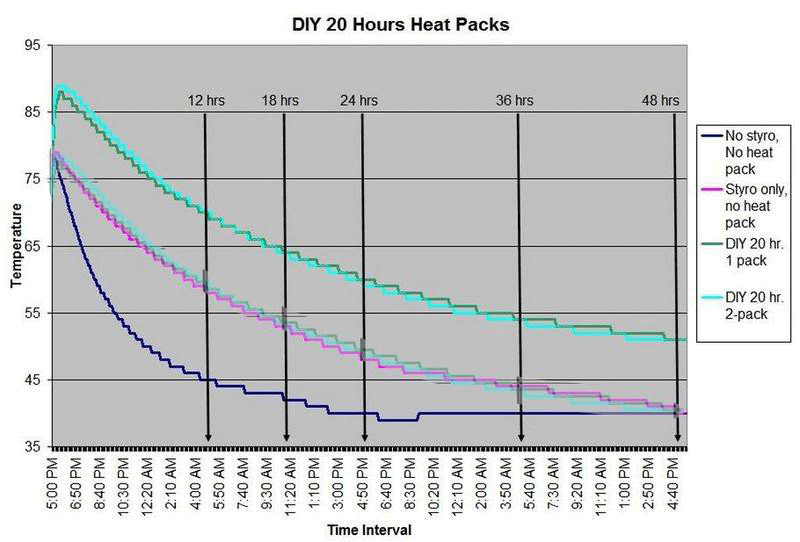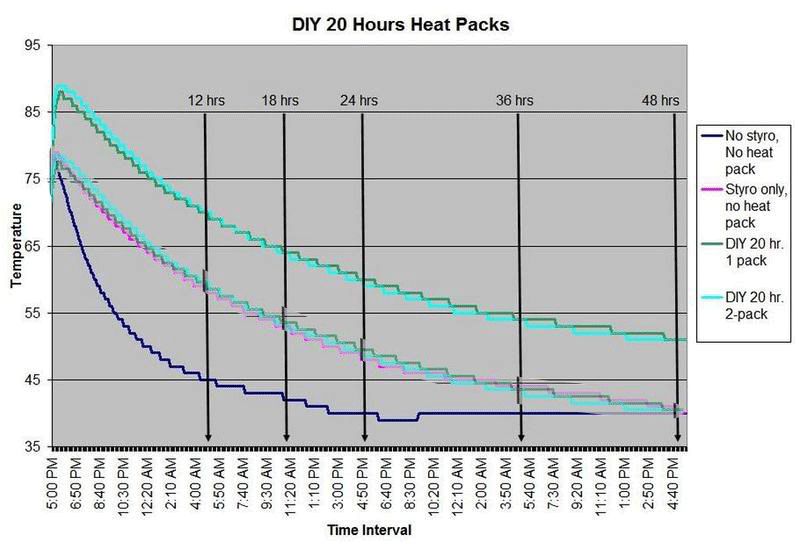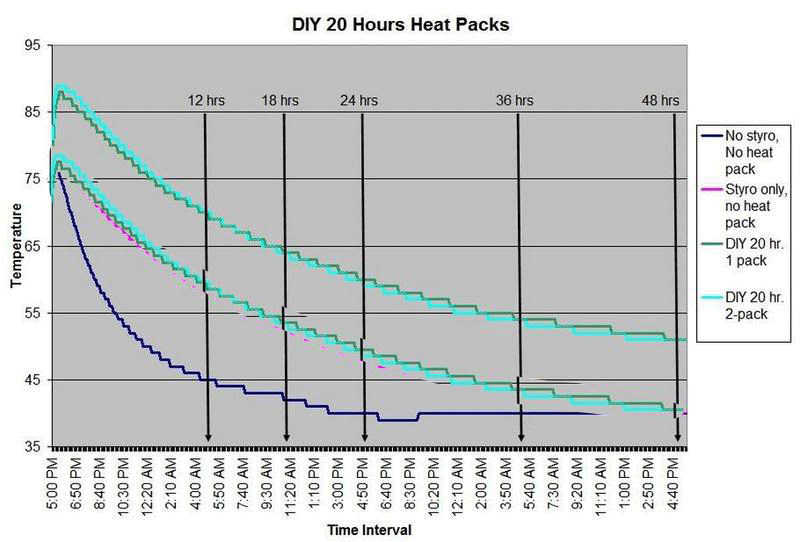
HEAT PACK STUDY: How efficient are heat packs when used to ship insulated boxes to maintain a warm temperature during the cold winter months in Michigan? Which heat packs should be used? What is the temperature of the water when received?
These are questions that have been on my mind when I ship coral frags during the winter months, so I designed a study to see how heat packs impact the shipped water temperature and the effectiveness of the insulated box. To design the study, many variables had to be controlled to make the proper comparisons meaningful, i.e. apples to apples. I used the same size and density Styrofoam containers, a uniform volume of water at a specific temperature and stored them in a controlled cold room at 4C +/- 1C (40F +/- 1F) degree. NIST calibrated temperature data loggers were sealed in plastic bags to keep them dry, then placed into the sealed water bag and recorded the water temperatures every 5 minutes for 48 hours.
Thank You
A special acknowledgement goes to Debbie Berlin (diyreef), Toddhttp://www.diyreef.com)/Todd Cherry (Cherry Corals) and Reefone for donating some different heat packs for testing and also for Jimsflies for helping me upload some of the data.
The Heat Pack Study follows below and in subsequent posts, however, I was having difficulty with the pdf files, so I have to convert the Excel charts to jpg format:
TEST CONTROLS:
First, every test must have some controls designed into the study for confidence in the results. Precision, accuracy, and blank samples are often used. For the purpose of precision, I started the study testing heat packs in triplicate to see if they were uniform in heating the water. After several tests on the Little Hotties, I concluded that testing in triplicate was no longer necessary as they were reproducible (precision). Accuracy is a little more difficult to show in this type of study, so the NIST calibration will have to suffice. In order to compare the results in a meaningful fashion, I prepared two blank controls, , blank meaning no heat packs. The first blank control was 27C bagged water with no Styrofoam box. The second blank was 27C bagged water packaged the same as the other tested boxes, but without any heat packs. These two blanks serve as control comparisons.
PACKAGING:
All packaging was uniform for every test (except the blank as described). A liter of 27C water was sealed with an activated temperature data logger in the bag, and then placed in the bottom of the Styrofoam cooler. A 2-ply newspaper was folded 4x and placed on the water bag as a barrier between the water and the heat packs. The heat pack(s) were taped to the inside of the lid and activated prior to use. Once assembled, a strip of tape was placed over the lid and sides of the box to make it secure, then placed in a temperature controlled environment. Shortly after 48 hours, the package was disassembled and the data logger downloaded to provide the readings and charts.
Next: Heat Packs Used
Since this information is still relevant, it may be helpful to review and open it to discussion.
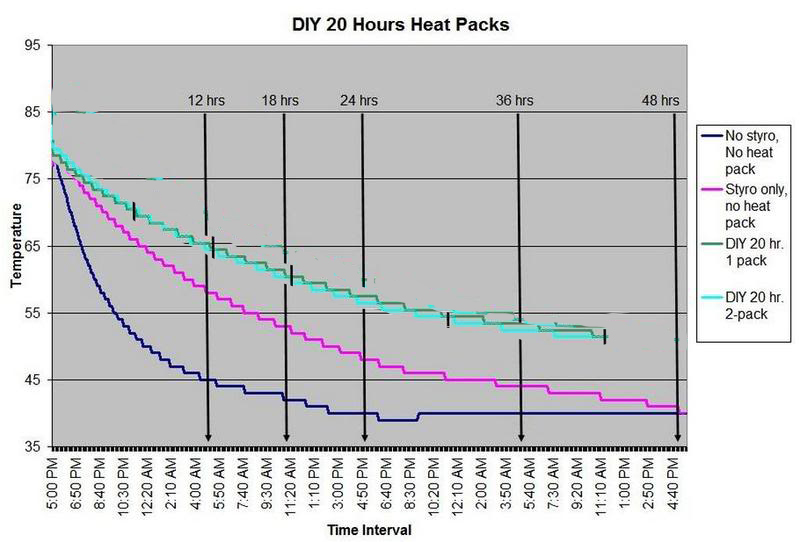
HEAT PACK STUDY: How efficient are heat packs when used to ship insulated boxes to maintain a warm temperature during the cold winter months in Michigan? Which heat packs should be used? What is the temperature of the water when received?
These are questions that have been on my mind when I ship coral frags during the winter months, so I designed a study to see how heat packs impact the shipped water temperature and the effectiveness of the insulated box. To design the study, many variables had to be controlled to make the proper comparisons meaningful, i.e. apples to apples. I used the same size and density Styrofoam containers, a uniform volume of water at a specific temperature and stored them in a controlled cold room at 4C +/- 1C (40F +/- 1F) degree. NIST calibrated temperature data loggers were sealed in plastic bags to keep them dry, then placed into the sealed water bag and recorded the water temperatures every 5 minutes for 48 hours.
Thank You
A special acknowledgement goes to Debbie Berlin (diyreef), Toddhttp://www.diyreef.com)/Todd Cherry (Cherry Corals) and Reefone for donating some different heat packs for testing and also for Jimsflies for helping me upload some of the data.
The Heat Pack Study follows below and in subsequent posts, however, I was having difficulty with the pdf files, so I have to convert the Excel charts to jpg format:
TEST CONTROLS:
First, every test must have some controls designed into the study for confidence in the results. Precision, accuracy, and blank samples are often used. For the purpose of precision, I started the study testing heat packs in triplicate to see if they were uniform in heating the water. After several tests on the Little Hotties, I concluded that testing in triplicate was no longer necessary as they were reproducible (precision). Accuracy is a little more difficult to show in this type of study, so the NIST calibration will have to suffice. In order to compare the results in a meaningful fashion, I prepared two blank controls, , blank meaning no heat packs. The first blank control was 27C bagged water with no Styrofoam box. The second blank was 27C bagged water packaged the same as the other tested boxes, but without any heat packs. These two blanks serve as control comparisons.
PACKAGING:
All packaging was uniform for every test (except the blank as described). A liter of 27C water was sealed with an activated temperature data logger in the bag, and then placed in the bottom of the Styrofoam cooler. A 2-ply newspaper was folded 4x and placed on the water bag as a barrier between the water and the heat packs. The heat pack(s) were taped to the inside of the lid and activated prior to use. Once assembled, a strip of tape was placed over the lid and sides of the box to make it secure, then placed in a temperature controlled environment. Shortly after 48 hours, the package was disassembled and the data logger downloaded to provide the readings and charts.
Next: Heat Packs Used