Tank - 150-gallon euro with ~20-gallon sump
Lights - Radion XR15 Pro x4, 9-hour for radion
Algae scrubber - the reverse of light schedule with a 60-minute overlap on each side
Dose 25.6 ML of BRS soda-ash via Neptune DOS every 3hrs +/-35%
Hybrid Sodium Hydroxide bubbler/CO2 (BRS Soda-lime media) (knicked name Hydro-Lime Scrubber) plumbed into skimmer - skimmer on 24/7
At night PH drops to 8.17, during the day PH has reached ~8.29
Tank located in basement approx. 3 feet from back door/bulkhead that is open often throughout the day in the spring and cracked about 1/2 in winter.
The basement is VERY airtight, 4 mil plastic moisture barrier fixed to concrete walls, 2x4 walls ALL with R-21 insulation, another 4 mil plastic moisture barrier, drywall, and that includes an R-30 insulation+drywall ceiling. No windows open as they are sealed and I have plans on replacing them this yr.
Alk- 8.86 to 9.2
Ca- 456
Mg- 1450
Inhabitants - fish load- 8 x Chromis, 3x Bimaculatus Anthias, 1 Naso. 1 Blue hippo
A mixed bag of sticks all growing very well.
Tank overall is doing well.
...Let me start by saying that I'm no scientist, I'm simply posting my observations and my engineering skills with implementing a hybrid approach to a Sodalime scrubber using @Randy Holmes-Farley information about Sodium Hydroxide and its ability to be more effective at scrubbing CO2.
The Hydro-lime scrubber that I created is a simple bucket filled with RO/DI water and about 40ml cup of Sodium Hydroxide, two air Stones along with an air pump introduce air into the solution - the unit has an airtight lid however it still allows for some air to be pulled in due to the holes drilled for the airlines. From there, an air hose to the CO2 reactor Inlet and then another airline from the CO2 reactors Outlet to my skimmer air Inlet. This is a good time to note that the Sodium Hydroxide bubbler/scrubber by itself was not able to remove/scrub enough CO2 to get my pH above ~8, but that is still a very excitable range.
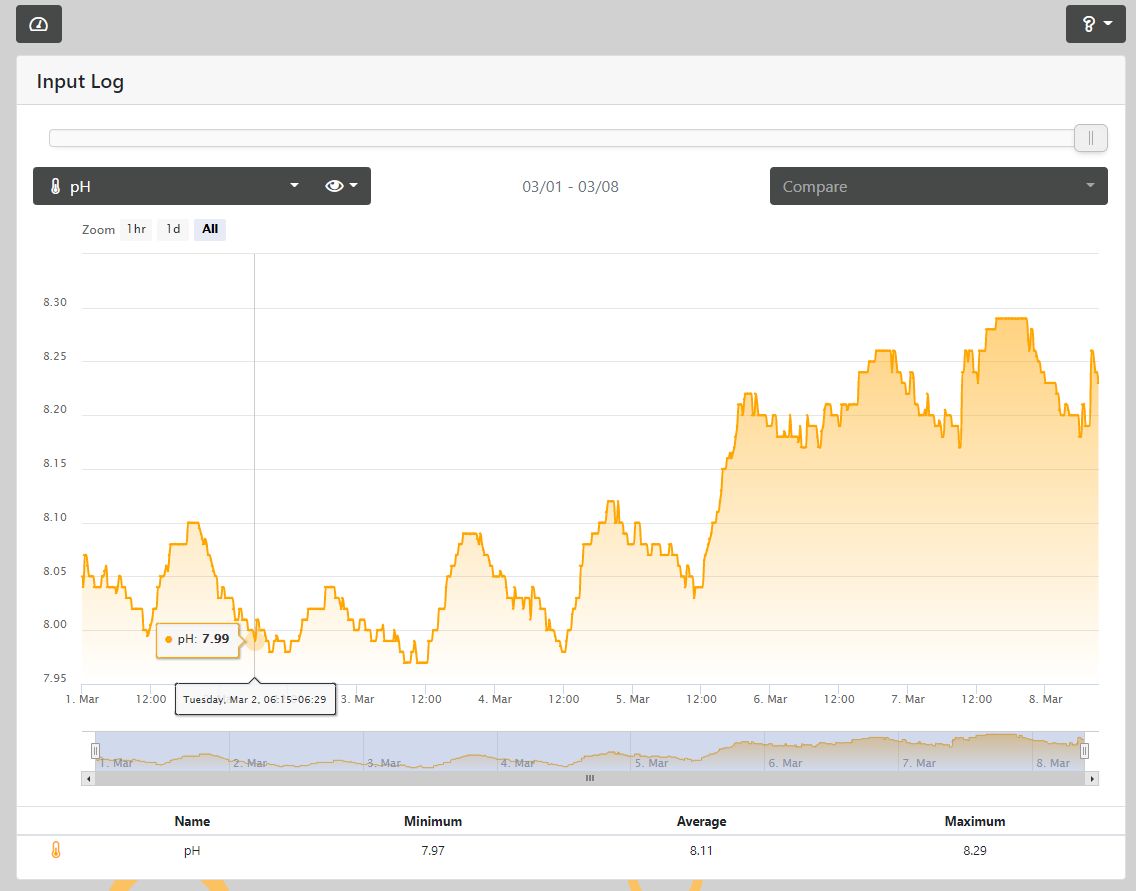
The main reason I decided to try this was that I was exhausting the Soda Lime media in a little over 2-1/2 days, I figured if I can get the incoming air to be less saturated with atmospheric CO2 prior to hitting the soda-lime it would last that much longer, or so I hoped. As of right now, I'm getting into the ~3-day mark and the soda-lime media shows no signs of being exhausted (yet). The added bonus of courses is the fact that my pH now reaches well above 8.20 (when the lights are on) and closer to that magic 8.3 some folks seem to obsess about.
The layout of the Hydro-Lime Scrubber.

...of course, only time will tell the longevity of this new (new to me) hybrid approach to scrubbing CO2 but I'm hopeful this will work out for me.
Lights - Radion XR15 Pro x4, 9-hour for radion
Algae scrubber - the reverse of light schedule with a 60-minute overlap on each side
Dose 25.6 ML of BRS soda-ash via Neptune DOS every 3hrs +/-35%
Hybrid Sodium Hydroxide bubbler/CO2 (BRS Soda-lime media) (knicked name Hydro-Lime Scrubber) plumbed into skimmer - skimmer on 24/7
At night PH drops to 8.17, during the day PH has reached ~8.29
Tank located in basement approx. 3 feet from back door/bulkhead that is open often throughout the day in the spring and cracked about 1/2 in winter.
The basement is VERY airtight, 4 mil plastic moisture barrier fixed to concrete walls, 2x4 walls ALL with R-21 insulation, another 4 mil plastic moisture barrier, drywall, and that includes an R-30 insulation+drywall ceiling. No windows open as they are sealed and I have plans on replacing them this yr.
Alk- 8.86 to 9.2
Ca- 456
Mg- 1450
Inhabitants - fish load- 8 x Chromis, 3x Bimaculatus Anthias, 1 Naso. 1 Blue hippo
A mixed bag of sticks all growing very well.
Tank overall is doing well.
...Let me start by saying that I'm no scientist, I'm simply posting my observations and my engineering skills with implementing a hybrid approach to a Sodalime scrubber using @Randy Holmes-Farley information about Sodium Hydroxide and its ability to be more effective at scrubbing CO2.
The Hydro-lime scrubber that I created is a simple bucket filled with RO/DI water and about 40ml cup of Sodium Hydroxide, two air Stones along with an air pump introduce air into the solution - the unit has an airtight lid however it still allows for some air to be pulled in due to the holes drilled for the airlines. From there, an air hose to the CO2 reactor Inlet and then another airline from the CO2 reactors Outlet to my skimmer air Inlet. This is a good time to note that the Sodium Hydroxide bubbler/scrubber by itself was not able to remove/scrub enough CO2 to get my pH above ~8, but that is still a very excitable range.
The main reason I decided to try this was that I was exhausting the Soda Lime media in a little over 2-1/2 days, I figured if I can get the incoming air to be less saturated with atmospheric CO2 prior to hitting the soda-lime it would last that much longer, or so I hoped. As of right now, I'm getting into the ~3-day mark and the soda-lime media shows no signs of being exhausted (yet). The added bonus of courses is the fact that my pH now reaches well above 8.20 (when the lights are on) and closer to that magic 8.3 some folks seem to obsess about.
The layout of the Hydro-Lime Scrubber.
...of course, only time will tell the longevity of this new (new to me) hybrid approach to scrubbing CO2 but I'm hopeful this will work out for me.


















