Very good point!!! I have to dose momentarily about 0.045ppm Po4 over the cause of the day, a single shot of so much P/Po4 will usually harm corals like a nutrient shock that many people have heard about and seen.Hello everybody!
A few hours offline, and im missing all the action in this thread ;-)
We do measure total phosphorus (via ICP-OES or ICP-MS, depending on which method the customer chooses) and also orthophosphate (PO4) using a photometric method based on the molybdenum blue/ascorbic acid method. We are using a Shimadzu Lab photometer with 4 cm optical path to have low detection limits. We do not calculate a phosphate value from the ICP data.
Phosphate is not very sensitive on ion chromatography (since it has a low specific conductivity), thus IC is not very useful for detection of phosphate in reef tanks. Photometry is the superior method in this case.
In case of higher phosphate levels total phosphorus and orthophosphate often agree very well - so most of the phosphorus in the sample is actually phosphate. This is not always the case, especially at lower nutrient levels, where a significant proportion of total phosphorus can be something else (might be oligophosphates or DNA or other phosphorus containing molecules).
We are using an orthophosphate based salt for phosphorus dosing, and i would be surprised if other products/brands would use other phosphorus sources. In my opinion it is important to dose phosphate spread out over several dposings per day, to achieve a constant availability. Otherwise this important nutrient might be depleted fast - either by consumption/metabolism, or by adsorption onto surfaces. Cycling between "available state" and "limitation" is imo stressful for corals, and should thus be avoided.
Best regards,
Christoph
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
ICP-MS measurement of Seawater Certified Reference Material
- Thread starter Christoph
- Start date
- Tagged users None
Yes, providing phosporus the same as all other products, only differing in the form provided. My guess, and other chemistry folk may have a better explanation, is that it allows the phosphorus to remain in the water column longer without it being precipitated onto a surface before the target audience, the tank inhabitants, can utilize it. I expect the reason it is not detectable is a limitation of the testing methods we use. We can only detect orthophosphate. Once the organics get stripped away from the organic form, then the phosphorus would be detectable after it gets converted to another form, likely PO4, phosphate.Which is the same as any phosphate product just in different form? What is the “benefit” of using an “organic” form over “inorganic?” Why is it not detectable initially, but becomes detectable later?
Is it possible that I’m polluting my system or do you think it’s being consumed by the rock, sand, corals, microfauna, etc?Yes, providing phosporus the same as all other products, only differing in the form provided. My guess, and other chemistry folk may have a better explanation, is that it allows the phosphorus to remain in the water column longer without it being precipitated onto a surface before the target audience, the tank inhabitants, can utilize it. I expect the reason it is not detectable is a limitation of the testing methods we use. We can only detect orthophosphate. Once the organics get stripped away from the organic form, then the phosphorus would be detectable after it gets converted to another form, likely PO4, phosphate.
What would cause it to be precipitated onto a surface and how long could this last?
Exactly what I’m doing, but just more.Very good point!!! I have to dose momentarily about 0.045ppm Po4 over the cause of the day, a single shot of so much P/Po4 will usually harm corals like a nutrient shock that many people have heard about and seen.
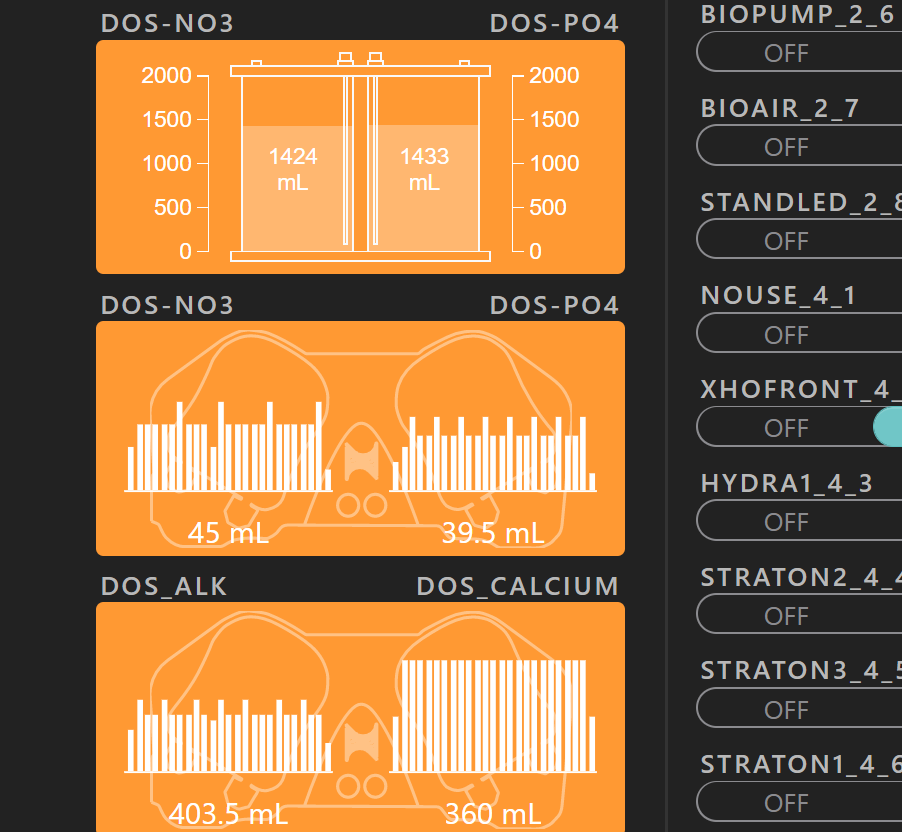

0.06 ppm daily currently. 60 X 0.1
P available every 25 minutes.
Old graph before I increased to 60x
The way to gauge if you are over doing it, is to periodically test your water column for phosphate. When you start to see a detectable reading that increases over time, then you may want to moderate your dosing.Is it possible that I’m polluting my system or do you think it’s being consumed by the rock, sand, corals, microfauna, etc?
What would cause it to be precipitated onto a surface and how long could this last?
I can't give you the chemistry definition of why phosphate precipitates onto surfaces. My explanation would be that when the concentration of phosphate in the water column exceeds the amount bound to the surface, some phosphate from the water column will precipitate to the surface.
The precipitated phosphate will remain bound to the surface until it either gets used, covered over with more aibiotic precipitation, or gets released back to the water column when the water column level drops low enough to cause the bound phosphate to release and bring the water column into equilibrium. So your surfaces act as a reservoir or phosphate storage.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,122
- Reaction score
- 63,461
ExactlyThe precipitated phosphate will remain bound to the surface until it either gets used, covered over with more aibiotic precipitation, or gets released back to the water column when the water column level drops low enough to cause the bound phosphate to release and bring the water column into equilibrium. So your surfaces act as a reservoir or phosphate storage.
Just tested and got 8 ppb. Was really happy!
Then tested again to make sure it wasn’t a fluke. ZERO.
Tomorrow morning if it’s still zero I’m increasing to .07 daily.
Then tested again to make sure it wasn’t a fluke. ZERO.
Tomorrow morning if it’s still zero I’m increasing to .07 daily.
Randy do you know of any examples of organic phosphate and why a company would go out of their way to source the organic product vs something readily assessable like Sodium Phosphate? Wouldn’t it be harder and more costly to obtain? There must be a clear advantage of using the organic form vs an inorganic form? Yes?? No??Relax. All methods reefers use detect the phosphate in sodium phosphate.
Seachem Fluorish phosphorus is also identical to sodium phosphate in form:inorganic orthophosphate.
Also, if the Hanna checker cannot detect organic phosphate how would the average reefer even know it’s in there without getting an ICP? Does it convert to inorganic phosphate after a period of time?
Sorry for all the questions. I’m really trying to wrap my brain around all of this.
- Joined
- Aug 24, 2016
- Messages
- 1,492
- Reaction score
- 2,281
I think the phosphorus - phosphate confusion is kind of funny, I'm sorry! 
Finally it is quite simple (and you can even find it in Wikipedia): Nearly all phosphorus in biochemistry and in the environment is phosphate, no matter whether organic or inorganic phosphate, all these phosphates are in the oxidation state +5.
Only a small proportion of phosphorus in biochemistry is phosphonates. However, at least one scientific article says, corals can also make use of phosphonates. This only to add some confusion.
Elemental phosphorus is virtually non existent, just like sodium as metal. Both are too reactive to exist as element or metal respectively. So we don't need to discuss phosphorus, for us all phosphorus is simply phosphate.
There are three kinds of phosphates, orthophosphate, inorganic polyphosphates (or pyrophosphates) and organic phosphates. While wet chemical tests without a prior step only find orthophosphate completely, ICP-analysis finds all kinds of phosphate compounds as phosphorus.
Polyphosphates and organic phosphates both differ from orthophosphate by having high-energy phosphate bonds. For wet chemical analysis these bonds have to be hydrolysed by a digestions step prior to normal orthophosphate analysis.
Inorganic polyphosphates are for example storage phosphates of algae and bacteria, but where also used as water softeners in washing detergents.
In oligotrophic reefs the polyphosphates and organic phosphates may be a significant proportion of total phosphate and exceed the orthophosphate concentration. Corals and many (most?) other organisms can make use of organic phosphates and inorganic polyphosphates by excreting enzymes, the alkaline phosphatases, that break down these phosphates to orthophosphate ready for uptake.
To make a long story short, it are all phosphates and corals can use them all for phosphate, but our test kits and photometers only find orthophosphate and in this way may underestimate the phosphate concentration, especially if phosphate concentrations are low and a high proportion of the phosphates are inorganic polyphosphates and organic phosphates of algae and bacteria.
Finally it is quite simple (and you can even find it in Wikipedia): Nearly all phosphorus in biochemistry and in the environment is phosphate, no matter whether organic or inorganic phosphate, all these phosphates are in the oxidation state +5.
Only a small proportion of phosphorus in biochemistry is phosphonates. However, at least one scientific article says, corals can also make use of phosphonates. This only to add some confusion.
Elemental phosphorus is virtually non existent, just like sodium as metal. Both are too reactive to exist as element or metal respectively. So we don't need to discuss phosphorus, for us all phosphorus is simply phosphate.
There are three kinds of phosphates, orthophosphate, inorganic polyphosphates (or pyrophosphates) and organic phosphates. While wet chemical tests without a prior step only find orthophosphate completely, ICP-analysis finds all kinds of phosphate compounds as phosphorus.
Polyphosphates and organic phosphates both differ from orthophosphate by having high-energy phosphate bonds. For wet chemical analysis these bonds have to be hydrolysed by a digestions step prior to normal orthophosphate analysis.
Inorganic polyphosphates are for example storage phosphates of algae and bacteria, but where also used as water softeners in washing detergents.
In oligotrophic reefs the polyphosphates and organic phosphates may be a significant proportion of total phosphate and exceed the orthophosphate concentration. Corals and many (most?) other organisms can make use of organic phosphates and inorganic polyphosphates by excreting enzymes, the alkaline phosphatases, that break down these phosphates to orthophosphate ready for uptake.
To make a long story short, it are all phosphates and corals can use them all for phosphate, but our test kits and photometers only find orthophosphate and in this way may underestimate the phosphate concentration, especially if phosphate concentrations are low and a high proportion of the phosphates are inorganic polyphosphates and organic phosphates of algae and bacteria.
Last edited:
I was going to inquire about the usage of organic phosphates in Plus NP, but I can't seem to find where I read that. I might be mixing it up with another product as the online literature does not mention it at all?
- Joined
- Aug 24, 2016
- Messages
- 1,492
- Reaction score
- 2,281
We say the nutrients in Plus-NP and NP-Bacto-Balance are bound by an "organic energy source", which basically only means "organic carbon dosing".
We use polyphosphates because they have better properties in solutions with high concentrations of calcium and magnesium ions and we use organic carbon to "package" some of the added nutrients as bacterial biomass that may be used by corals directly or go into the food web and nutrient cycles. These are the two reasons why phosphates added with Plus-NP may be "hidden" for some time.
We use polyphosphates because they have better properties in solutions with high concentrations of calcium and magnesium ions and we use organic carbon to "package" some of the added nutrients as bacterial biomass that may be used by corals directly or go into the food web and nutrient cycles. These are the two reasons why phosphates added with Plus-NP may be "hidden" for some time.
Randy, can you educate me on organic forms of phosphate that are commonly available or dosed. I believe Trisodium Phosphate (TSP) is inorganic (which I’ve been dosing for a while). I’d like to try an organic form to see if I can observe a difference.you can dose organic or inorganic phosphate, if you want to dose, but must folks elect to dose inorganic phosphate, such as sodium phosphate.
@Hans-Werner great to have you on the thread!We say the nutrients in Plus-NP and NP-Bacto-Balance are bound by an "organic energy source", which basically only means "organic carbon dosing".
We use polyphosphates because they have better properties in solutions with high concentrations of calcium and magnesium ions and we use organic carbon to "package" some of the added nutrients as bacterial biomass that may be used by corals directly or go into the food web and nutrient cycles. These are the two reasons why phosphates added with Plus-NP may be "hidden" for some time.
What would be your Phosphate / Phosphorus supplement of choice if you were seeing corals starving or looking very pale and you wanted to boost “only” the PO4 in the water column.?
Finally getting some great clarification!I think the phosphorus - phosphate confusion is kind of funny, I'm sorry!
Finally it is quite simple (and you can even find it in Wikipedia): Nearly all phosphorus in biochemistry and in the environment is phosphate, no matter whether organic or inorganic phosphate, all these phosphates are in the oxidation state +5.
Only a small proportion of phosphorus in biochemistry is phosphonates. However, at least one scientific article says, corals can also make use of phosphonates. This only to add some confusion.
Elemental phosphorus is virtually non existent, just like sodium as metal. Both are too reactive to exist as element or metal respectively. So we don't need to discuss phosphorus, for us all phosphorus is simply phosphate.
There are three kinds of phosphates, orthophosphate, inorganic polyphosphates (or pyrophosphates) and organic phosphates. While wet chemical tests without a prior step only find orthophosphate completely, ICP-analysis finds all kinds of phosphate compounds as phosphorus.
Polyphosphates and organic phosphates both differ from orthophosphate by having high-energy phosphate bonds. For wet chemical analysis these bonds have to be hydrolysed by a digestions step prior to normal orthophosphate analysis.
Inorganic polyphosphates are for example storage phosphates of algae and bacteria, but where also used as water softeners in washing detergents.
In oligotrophic reefs the polyphosphates and organic phosphates may be a significant proportion of total phosphate and exceed the orthophosphate concentration. Corals and many (most?) other organisms can make use of organic phosphates and inorganic polyphosphates by excreting enzymes, the alkaline phosphatases, that break down these phosphates to orthophosphate ready for uptake.
To make a long story short, it are all phosphates and corals can use them all for phosphate, but our test kits and photometers only find orthophosphate and in this way may underestimate the phosphate concentration, especially if phosphate concentrations are low and a high proportion of the phosphates are inorganic polyphosphates and organic phosphates of algae and bacteria.
Thank you for clarifying that Dr. Balling! You and Randy Farley have really helped me to understand this at a much deeper level.
I’m glad this is finally put to rest. We are essentially dosing Phosphate to our reef tanks and testing/measuring inorganic Phosphate with our home test kits. Only ICP can measure the atom Phosphorus.Elemental phosphorus is virtually non existent, just like sodium as metal. Both are too reactive to exist as element or metal respectively. So we don't need to discuss phosphorus, for us all phosphorus is simply phosphate
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,122
- Reaction score
- 63,461
Randy, can you educate me on organic forms of phosphate that are commonly available or dosed. I believe Trisodium Phosphate (TSP) is inorganic (which I’ve been dosing for a while). I’d like to try an organic form to see if I can observe a difference.
Well, the most common form of organic phosphate added to reef tanks is food. It is filled with organic phosphate.
Beyond that, I am not aware of what products might contain organic phosphate. It is going to be substantially more expensive. I'm also not aware of what molecules people might pick.
Do yo have reason to think any products add it?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,122
- Reaction score
- 63,461
I explain the different forms of phosphate in a reef tank here:
for example:
Organic Phosphate
In seawater, organic phosphorus compounds are far more varied and complex than inorganic phosphate. Many common biochemicals contain phosphorus and every living cell contains a wide variety of them. Molecules such as DNA (deoxyribonucleic acid), ATP (adenosine triphosphate), phospholipids (e.g., lecithin) and many proteins contain phosphate groups. In these molecules, the basic phosphate structure is covalently attached to the remainder of the organic molecule through one or more phosphate ester bonds to a carbon atom.
These bonds are stable for some period of time in water, but eventually break down to release inorganic orthophosphate from the molecule’s organic part, a process that can be sped up through the action of enzymes in a reef aquarium. Many of these organic phosphate compounds will be readily removed from an aquarium by skimming. Export of organic phosphates is likely the major way that skimming can reduce inorganic orthophosphate levels in an aquarium. Orthophosphate ions are not significantly removed by skimming because they do not adsorb onto an air/water interface. but many organic phosphates can be removed by skimming or other organic export methods. These other methods include granular activated carbon (GAC) and polymeric binders such as Purigen or a Polyfilter, which remove the ions before they are broken down into inorganic orthophosphate.
An important point about organic phosphates is that most of them are not readily bound by inorganic phosphate-binding materials used in the aquarium hobby. Consequently, while these products may do a fine job of reducing inorganic orthophosphate, they may not substantially reduce organic phosphates.
A final point is that organic phosphates are not detected by most test kits designed for hobbyists. Those that do detect organic phosphates, e.g., Hach PO-24, break the phosphate off the organic compound, thereby converting it into inorganic orthophosphate prior to testing. These kits are tedious to use and expensive, however, so they’re not for every hobbyist. Indeed, I’ve never used one.
Testing by ICP (inductively coupled plasma) by a commercial enterprise such as Triton or ENC Labs will give a value for total phosphorus, including both organic and inorganic forms. Depending on how the sample is prepared and processed, the organic forms may be individual molecules dissolved in the water, or larger aggregates, all the way up to and including whole bacteria (although Triton says these are removed before testing). Now that larger numbers of reef aquarists are getting ICP testing done, it does not appear that total phosphorus/phosphate is noticeably higher than what those same reefers are getting with test kits, so the organic forms may often be a minor contributor to total phosphate in many reef aquaria.
Organics in seawater are often measured in terms of their nitrogen content, such as dissolved organic nitrogen (DON) and particulate organic nitrogen (PON). The same is true for phosphorus, using the terms dissolved organic phosphorus (DOP) and particulate organic phosphorus (POP). Table 1 shows the relative concentrations of C, N and P in typical dissolved organic material found in seawater.1 In dissolved organic material, nitrogen is about tenfold less prevalent than carbon, and phosphorus is several hundredfold lower in concentration than carbon.

Phosphate in the Reef Aquarium
The phosphorus atom is one of living matter's basic building blocks. It is present in every living creature and in every reef aquarium's water. Unfortunately, it is often present in excess in reef aquaria, and that excess has the potential to cause at least two substantial problems for...
www.reefedition.com
for example:
Organic Phosphate
In seawater, organic phosphorus compounds are far more varied and complex than inorganic phosphate. Many common biochemicals contain phosphorus and every living cell contains a wide variety of them. Molecules such as DNA (deoxyribonucleic acid), ATP (adenosine triphosphate), phospholipids (e.g., lecithin) and many proteins contain phosphate groups. In these molecules, the basic phosphate structure is covalently attached to the remainder of the organic molecule through one or more phosphate ester bonds to a carbon atom.
These bonds are stable for some period of time in water, but eventually break down to release inorganic orthophosphate from the molecule’s organic part, a process that can be sped up through the action of enzymes in a reef aquarium. Many of these organic phosphate compounds will be readily removed from an aquarium by skimming. Export of organic phosphates is likely the major way that skimming can reduce inorganic orthophosphate levels in an aquarium. Orthophosphate ions are not significantly removed by skimming because they do not adsorb onto an air/water interface. but many organic phosphates can be removed by skimming or other organic export methods. These other methods include granular activated carbon (GAC) and polymeric binders such as Purigen or a Polyfilter, which remove the ions before they are broken down into inorganic orthophosphate.
An important point about organic phosphates is that most of them are not readily bound by inorganic phosphate-binding materials used in the aquarium hobby. Consequently, while these products may do a fine job of reducing inorganic orthophosphate, they may not substantially reduce organic phosphates.
A final point is that organic phosphates are not detected by most test kits designed for hobbyists. Those that do detect organic phosphates, e.g., Hach PO-24, break the phosphate off the organic compound, thereby converting it into inorganic orthophosphate prior to testing. These kits are tedious to use and expensive, however, so they’re not for every hobbyist. Indeed, I’ve never used one.
Testing by ICP (inductively coupled plasma) by a commercial enterprise such as Triton or ENC Labs will give a value for total phosphorus, including both organic and inorganic forms. Depending on how the sample is prepared and processed, the organic forms may be individual molecules dissolved in the water, or larger aggregates, all the way up to and including whole bacteria (although Triton says these are removed before testing). Now that larger numbers of reef aquarists are getting ICP testing done, it does not appear that total phosphorus/phosphate is noticeably higher than what those same reefers are getting with test kits, so the organic forms may often be a minor contributor to total phosphate in many reef aquaria.
Organics in seawater are often measured in terms of their nitrogen content, such as dissolved organic nitrogen (DON) and particulate organic nitrogen (PON). The same is true for phosphorus, using the terms dissolved organic phosphorus (DOP) and particulate organic phosphorus (POP). Table 1 shows the relative concentrations of C, N and P in typical dissolved organic material found in seawater.1 In dissolved organic material, nitrogen is about tenfold less prevalent than carbon, and phosphorus is several hundredfold lower in concentration than carbon.
I don’t.Well, the most common form of organic phosphate added to reef tanks is food. It is filled with organic phosphate.
Beyond that, I am not aware of what products might contain organic phosphate. It is going to be substantially more expensive. I'm also not aware of what molecules people might pick.
Do yo have reason to think any products add it?
Also, one last question about products that say phosphorus on the label. Do you suspect these are just sodium phosphate, potassium phosphate, etc.? Or something else?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,122
- Reaction score
- 63,461
I don’t.
Also, one last question about products that say phosphorus on the label. Do you suspect these are just sodium phosphate, potassium phosphate, etc.? Or something else?
I think so. Some may be a carry over from fertilizer legal requirements for labeling.
I just realized Seachem Flourish “Phosphorus” is “potassium phosphate.” They say it on the bottle.



Last edited:
Let me ask you this…
Is it possible to balance Phosphorus with Phosphate? I’ve noticed a lot of ICP analysis reveal a ratio that appears to be unbalanced on most tanks according to the normal levels provided by the analysis.
If so are there two different products you can purchase to balance out the ratio better? Are some Phosphate/ Phosphorus forms or compounds more bioavailable than others? What is the best available form for the corals and other biology to uptake quickly and easily?
Lets take a quick look at a few:
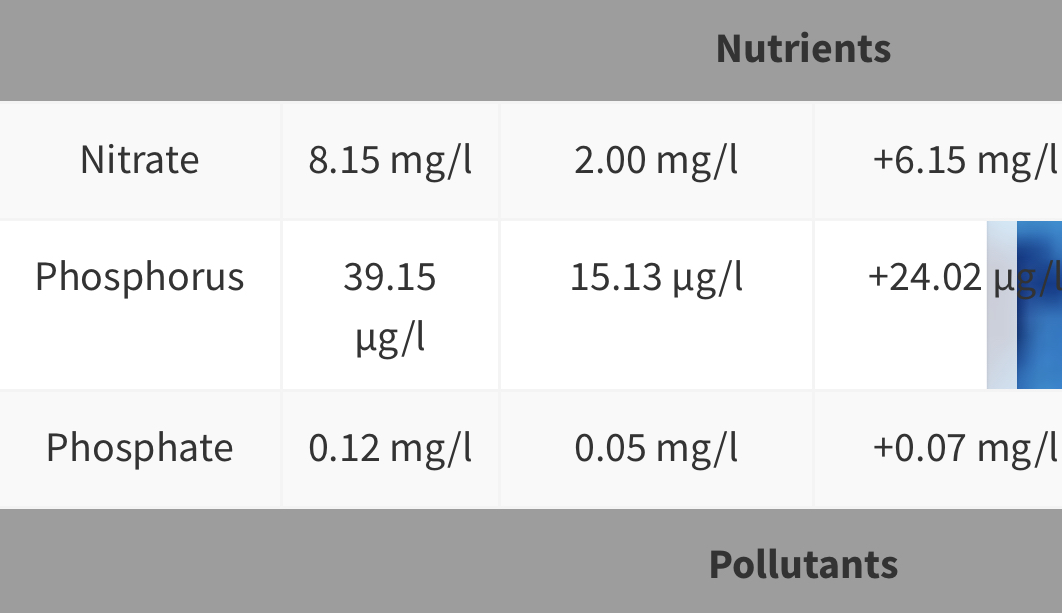
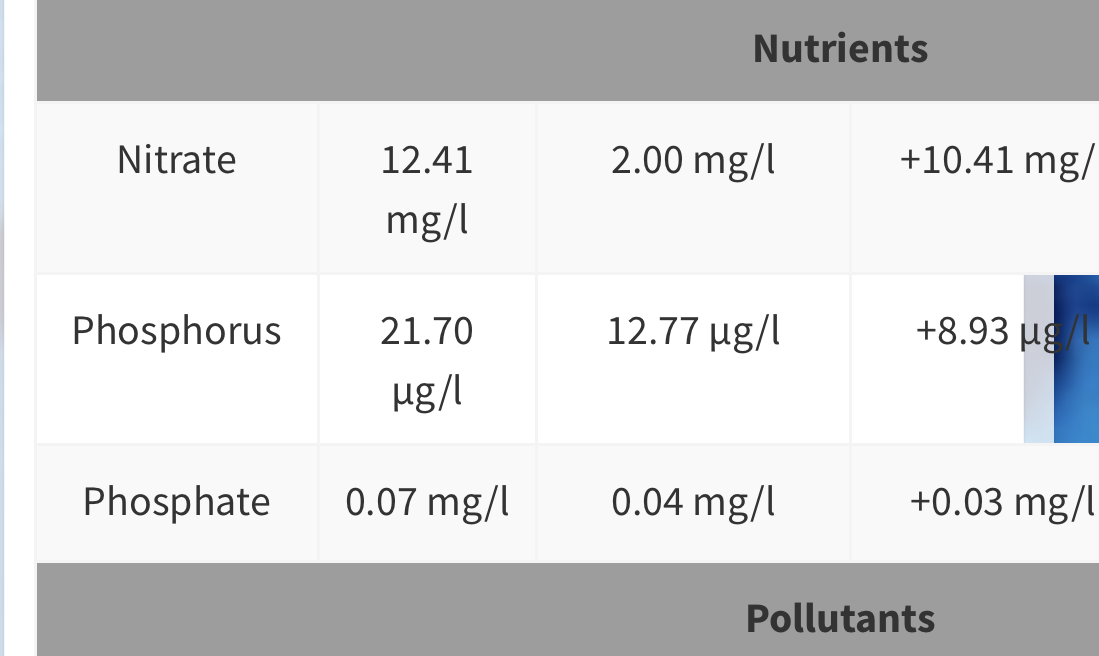
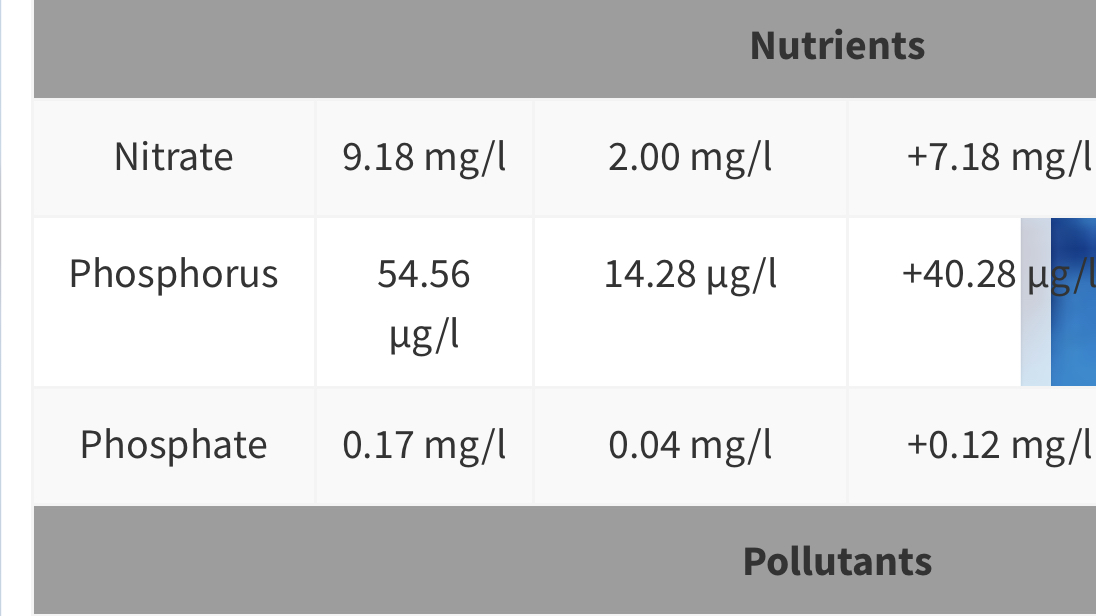
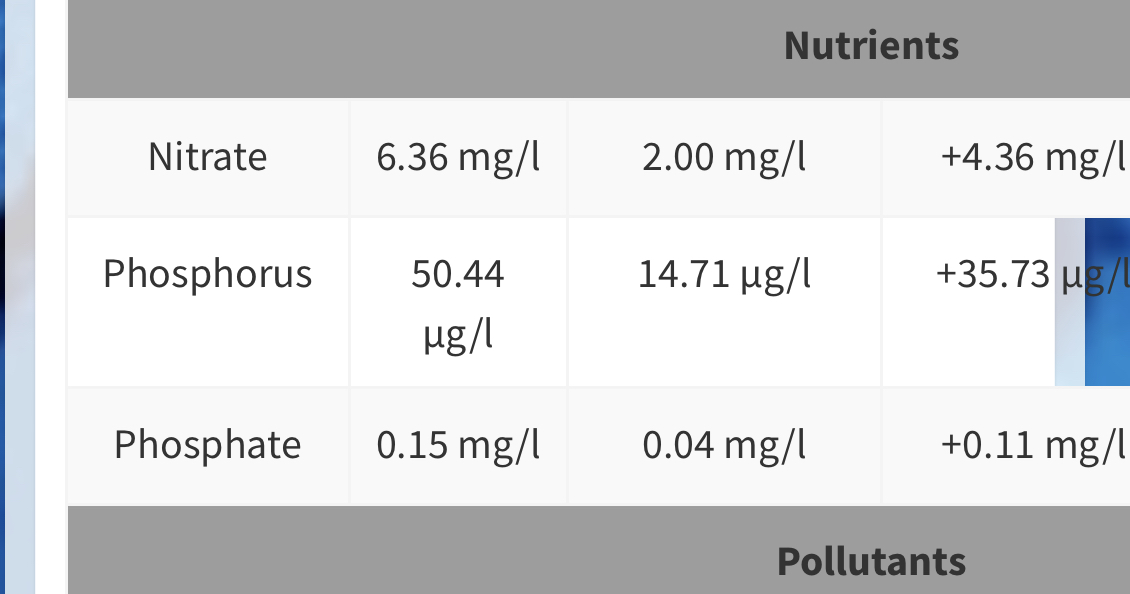
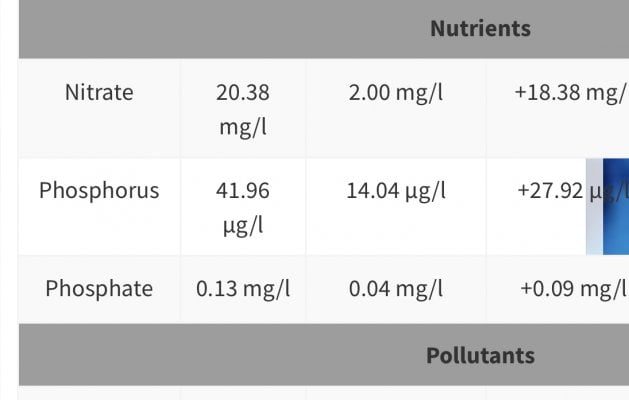
This one is my last ICP.
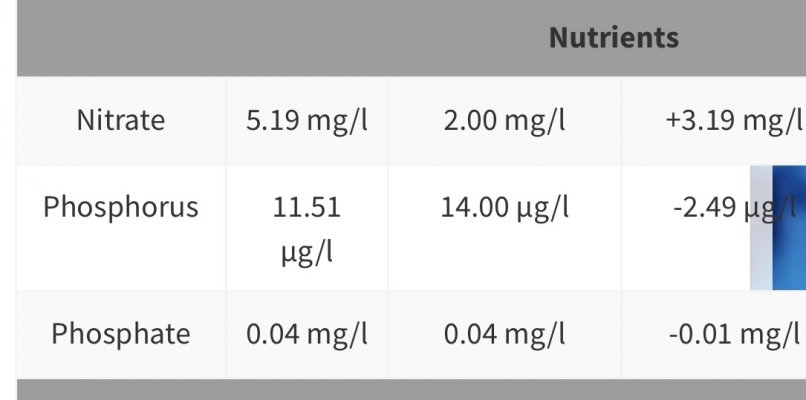
Is it possible to balance Phosphorus with Phosphate? I’ve noticed a lot of ICP analysis reveal a ratio that appears to be unbalanced on most tanks according to the normal levels provided by the analysis.
If so are there two different products you can purchase to balance out the ratio better? Are some Phosphate/ Phosphorus forms or compounds more bioavailable than others? What is the best available form for the corals and other biology to uptake quickly and easily?
Lets take a quick look at a few:

This one is my last ICP.

Last edited:
Similar threads
- Replies
- 49
- Views
- 2,623
- Replies
- 5
- Views
- 217
- Replies
- 18
- Views
- 1,640
New Posts
-
*** TUNZE PREMIUM GLASS CARE GIVEAWAY!!! *** Two Prizes for Two Winners!!!
- Latest: reefedandconfused
-


















