Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,344
- Reaction score
- 63,688
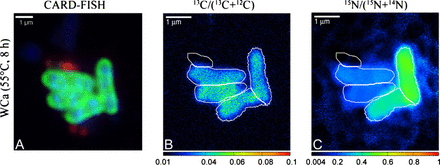
Corals in their natural environment are dissolved organic carbon (DOC) net positive. About 40% of the photosynthetically fixed carbon by zoox is excreted by the coral in the surrounding water as mucus. I would expect it is like this in the aquariums too. Corals will probably take some of the carbon dosed as acetate but will release back much more as mucus. Actually this could be the reason why I dont need to dose organic carbon to lower nitrates and phosphates in my tank full of corals - the corals themselves are dosing enough
Yes, I can believe that they are net excretors, at least in the absence of dosing readily metabolized organics such as acetate where we push those to unusually high levels.