The creators of vibrant said it won’t reproduce in seawater.How so?
Are you talking about testing done by somebody else?
I grew stuff from vibrant bottles in Instant Ocean at 1.026 s.g. As long as enough food was added (fish flake or LB broth), I got response from each product.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
My speculation: Vibrant has some fluconazole in it...
- Thread starter ScottB
- Start date
- Tagged users None
- Joined
- May 22, 2016
- Messages
- 6,525
- Reaction score
- 10,058
Can you point me to that statement?The creators of vibrant said it won’t reproduce in seawater.
Depending on context, it could be a reference to the amount of nutrients in seawater being too low, or it could mean that whatever is saltwater-culturable in the bottle are irrelevant strains.
Thank you. I tried looking through @UWC ’s thread but I can’t find that statement.How so?
Are you talking about testing done by somebody else?
I grew stuff from vibrant bottles in Instant Ocean at 1.026 s.g. As long as enough food was added (fish flake or LB broth), I got response from each product.
Under Water Creations: Did you ever state that vibrant will not reproduce in our tanks?
- Joined
- Jul 18, 2019
- Messages
- 330
- Reaction score
- 542
So here's the problem with this whole "doesn't reproduce in seawater thing". Having worked with natural products purification before, we have to grow things to really high OD to get any useful amount of product, and yet these things all come nearly clear as water and yet are effective. So this either means the bacteria grow in water or whatever is the active ingredient has nothing to do with the bacteria or thirdly, they culture up the bacteria and send us the culture liquid after spinning down. However, because the liquid we receive is clear, I don't think this is the case. Some thing seems pretty fishy here....
Thank you for sharing this data. The one for me that sticks out is agrobacterium which is a soil bacteria that I routinely work with to use as a plant pathogen...but it is a minority of the consortia...I suppose the best thing to do is to refer to the top 10 hits in abundance as these are probably the major constituents of the products. Nitrareductor sp. also seems like a pretty good candidate for something being active since....well the name implies some type of nitrogen reduction capability and apparently is grown for sludge removal. Not sure what to make the aeromonad since for us these are normally environmental contaminants or pathogens. Is it possible to get the actual reads for the NGS data from the company? I'm curious if something better can be found outside of just a BLAST of the sequence data.I'll go ahead and post this since we've attracted some sophisticated eyeballs that can appreciate interesting data even if it doesn't answer the questions.
Here's the aquabiomics list of sequenced microbes that I got back from culturing up 7 popular hobby bacterial additives for consuming organics (not nitrifiers). Vibrant was included, though I debated whether it even fit, but it cultured up and so I kept it in.
First, here's the culture-up data showing that there were nice cloudy samples of similar density from each product, so something that grew from each product bottle ought to have been well-sampled by the aquabiomics data.
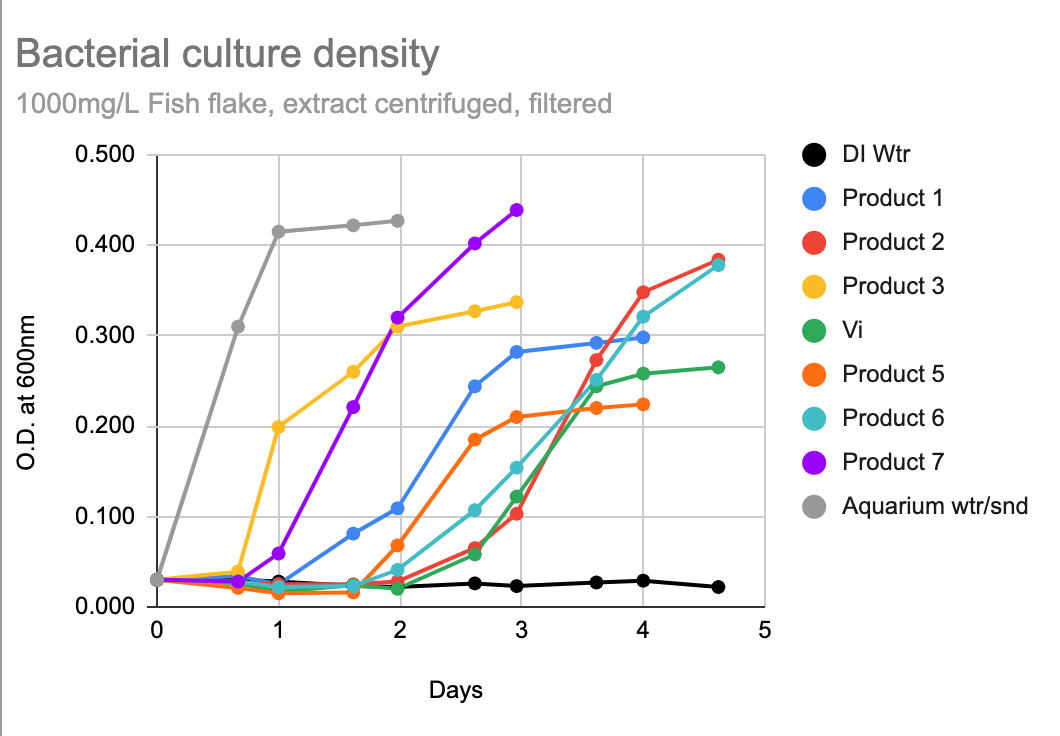
And below is the aquabiomics data. I pooled the cloudy solutions that were grown from each bottle, mixed them and used that as the aquabiomics sample. They ran it as an unknown culture, just looking for all the strains detectable. (I sampled what grew from my aquarium sand/water in a separate test - there were zero organisms that overlapped between the cultured bottle products and the stuff from my aquarium.)
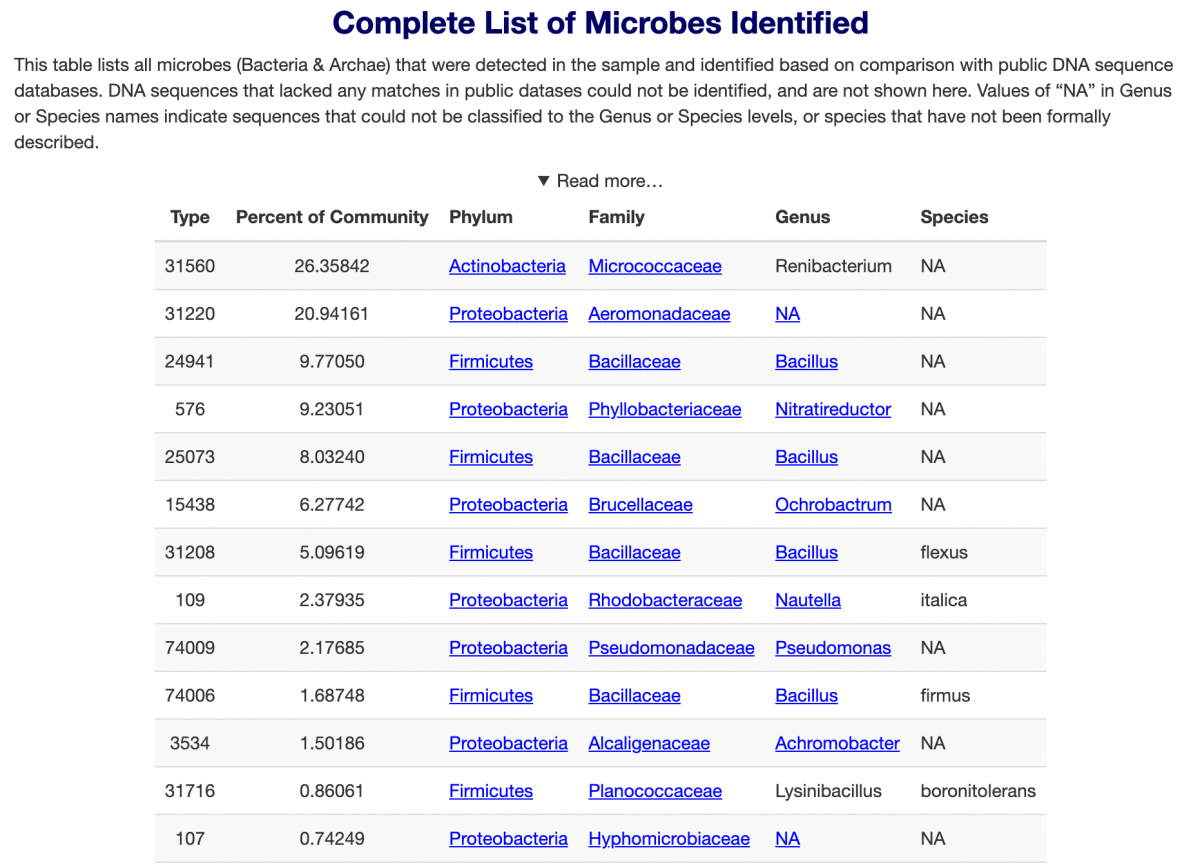
continued...
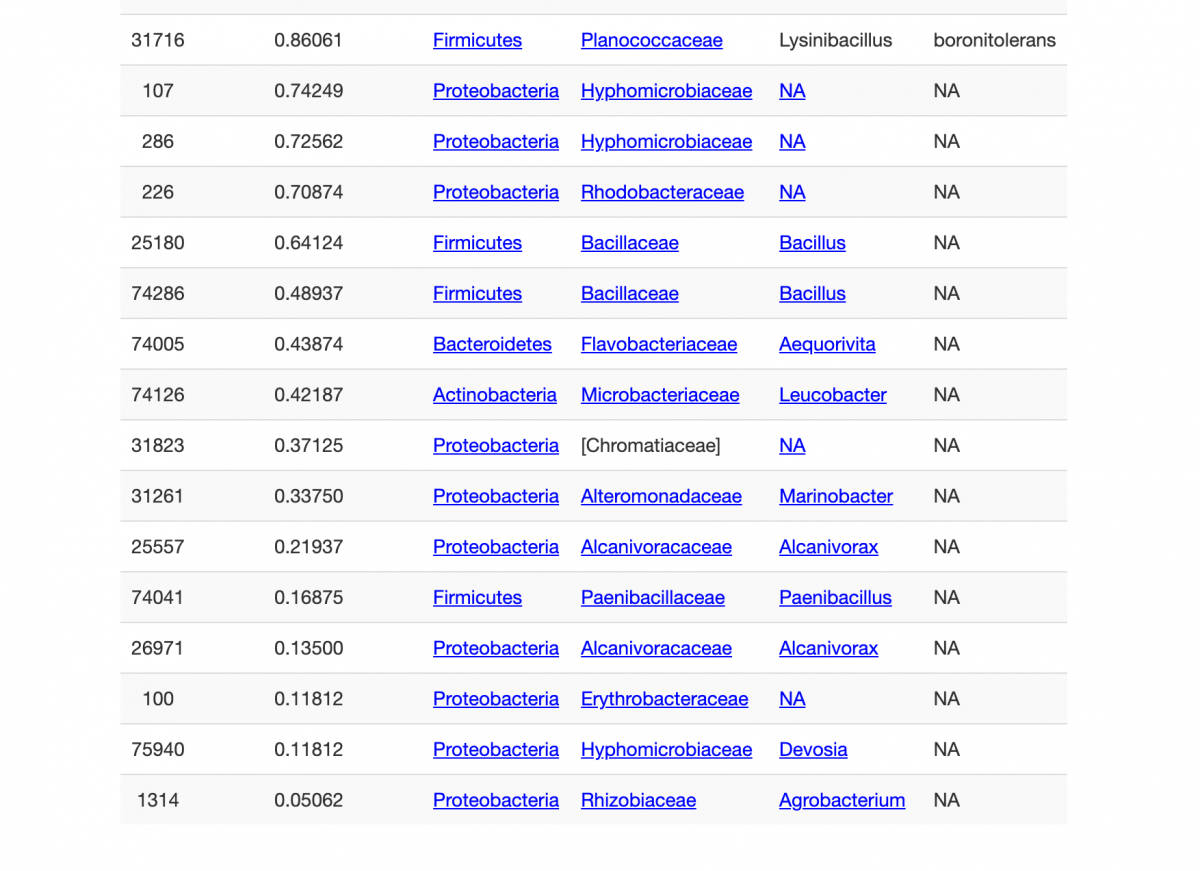
Some things here are what ought to be expected - lots of Bacillus. But there are other things that probably nobody is intending to be there. More diversity (27 strains) was cultured from the 7 bottles than I expected.
Does this confirm an algicidal bacteria in vibrant? nope. At least nothing that I see.
It it possible there's a relevant strain of bacteria from vibrant in this list? sure. it's possible. For instance multiple strains of Bacillus are not ID'd below the genus, and a quick google scholar search for bacillus algicide brings thousands of hits on known algicidal effects within that enormous genus.
(way outside my comfort zone here. Would welcome commentary from others.)
- Joined
- May 22, 2016
- Messages
- 6,525
- Reaction score
- 10,058
Some aspects of what people see from vibrant can't be due to bacterial activity coming out of the bottle based on the timeline.Having worked with natural products purification before, we have to grow things to really high OD to get any useful amount of product, and yet these things all come nearly clear as water and yet are effective. So this either means the bacteria grow in water or whatever is the active ingredient has nothing to do with the bacteria or thirdly, they culture up the bacteria and send us the culture liquid after spinning down. However, because the liquid we receive is clear, I don't think this is the case.
For instance, people note clarity increases in very short time ~1hr. The culture-ups from these products take days to get detectable growth.
Also, a hanna PO4 test on 4mL vibrant / Liter tankwater causes the test to fail. The blue/gray product that the test forms precipitates and clumps, falling out of solution. So obviously some chemical properties of the product are in the bottle already, irrespective of what bacteria are in there and what they may do upon addition to the tank.
I will request the raw data from the aquabiomics test and see if that has anything of further interest.
Thanks for the ideas.
- Joined
- May 22, 2016
- Messages
- 6,525
- Reaction score
- 10,058
I wouldn't necessarily discount the low-population organisms. I cultured these up on the soluble/suspended bits of boiled fish flake material because I was looking for eaters of organics. So if a useful plant-pathogenic bacteria appears in low concentration, it may be a relevant bacteria that was just much less suited to the culture-up conditions.The one for me that sticks out is agrobacterium which is a soil bacteria that I routinely work with to use as a plant pathogen...but it is a minority of the consortia...I suppose the best thing to do is to refer to the top 10 hits in abundance as these are probably the major constituents of the products.
I would add that while it might be useful, even species level sequencing may not be terribly effective due to drastic differences in at the strain level. Now I don’t expect the product to have that much R&D but for example I worked with, among other organisms, lactobacillus brevis, plantarum, and such for commercial products and you can have strains with totally different effects. We being a private company would full length sequence the genomes of strains showing what we were after, which were quite hard to find over the years, and the sequence while matching the ribosomal dna for species id, drastically differed in some cases. There were times the genes were present but inactive, others where they had huge insertions or deletions of DNA and none matched published data.
RNAseq which for those that don’t know, is mass sequencing of the RNA which would be the next step to see what compounds are being expressed combined with the population sequencing already done. But it’s probably a great deal of overkill for this.
Basically im saying this can be very complex to sort out, it might not be, but it can.
side note : It’s a reason to really trust a company that just says they add probiotics even if they list the species as it’s really the strain that is important. USA labeling isn’t as strict as other counties - most of our hard data was for approval in the EU and Canada, and in the USA additives for animal feed are harder to get label approval than for humans.
RNAseq which for those that don’t know, is mass sequencing of the RNA which would be the next step to see what compounds are being expressed combined with the population sequencing already done. But it’s probably a great deal of overkill for this.
Basically im saying this can be very complex to sort out, it might not be, but it can.
side note : It’s a reason to really trust a company that just says they add probiotics even if they list the species as it’s really the strain that is important. USA labeling isn’t as strict as other counties - most of our hard data was for approval in the EU and Canada, and in the USA additives for animal feed are harder to get label approval than for humans.
- Joined
- Jul 18, 2019
- Messages
- 330
- Reaction score
- 542
We are just about to submit a deep amplicon run so I guess it would have technically been free to do since we pay per plate and not sample. Don’t have time to do the library prep unfortunately!Sounds like at least three of us have access to a Miseq... Someone just needs to do it!
- Joined
- May 22, 2016
- Messages
- 6,525
- Reaction score
- 10,058
hmmm this one looks fun. Might poke around and see what I find.This might be worth trying: https://pubmed.ncbi.nlm.nih.gov/33191523/ should(conjecture) detect if quat like algaefix is in the mixture.
- Joined
- Dec 28, 2016
- Messages
- 22,709
- Reaction score
- 21,894
I do not think Vibrant contains fluconazole - since you can use activated carbon with it - and fluconazole should be rapidly removed.How did I come up with this theory? Allow me to explain.
About the only nuisance pest/algae I have not had is bryopsis in any of my tanks, but I have used fluconazole in other bryopsis overrun displays a few times. My LFS services many tanks so they have considerable experience with it as well. Most outcomes were quite good. A very small handful of very bad outcomes. That is covered in other threads, so I will leave that debate aside.
Vibrant I have dosed a small handful of times in my own display to help manage this or that, when my herbivore population has gone on strike, or I get some bubble algae. I never dose it higher than the suggested levels for a fairly "clean" tank. My experience with it is generally pretty good, but I am very cautious and patient with it.
Early Summer, I removed a bunch of my old rock from my display as it was overrun with encrusting SPS plus a bizarre vermetid outbreak. I built some nice structures with dead rock, made a modest effort to cure it, and chucked it in. Well, you know what happened then. Got GHA all over it within a couple months. CUC and herbivores weren't getting it done, half the tank looked terrible, so I started in with some Vibrant, and now maybe 5-6 weeks in with it.
-- All of the GHA that is exposed to my lights got weak and died out.
-- All of the GHA that is shaded, seems rather content.
If you follow the fluconazole thread, you will see that this is also how fluconazole behaves. The cellular disruption process (as described by Jose Mayo) requires active photosynthesis to occur. Any bryopsis (or GHA) that is shaded remains in tact. I am aware that Vibrant is marketed as a "blend of bacteria". Given that it does tend to lower nutrients gradually over time suggests to me that it does have some bacteria in it.
WHAT DO YOU THINK ABOUT MY THEORY?
To me, this is a smoking gun. Like many/most(?) of you in the SPS forum, I love me my acropora and the risk -- however small -- of a bad outcome for my expensive tenuis is just not worth it. I will just have to let this algae phase pass on its own.
Two pics. The first is my (now) pristine rock that is fully exposed. The second pic is my happy GHA, hidden under an overhang.


- Joined
- May 22, 2016
- Messages
- 6,525
- Reaction score
- 10,058
Wanted to follow up on this after playing around with it for a bit.
from the paper, "This study investigates the utility of bromophenol blue (BPB) as a safe, rapid and user-friendly indicator to detect in situ residual QACs [quaternary ammonium compounds] dried on hard, non-porous surfaces, as well a means to assess their antimicrobial efficacy. At pH 7, BPB has a purple colour which turns blue upon its complexation with QACs..."
So I dried drops of a few different things on glass slides and then applied a drop of bromophenol blue to the residue of each.
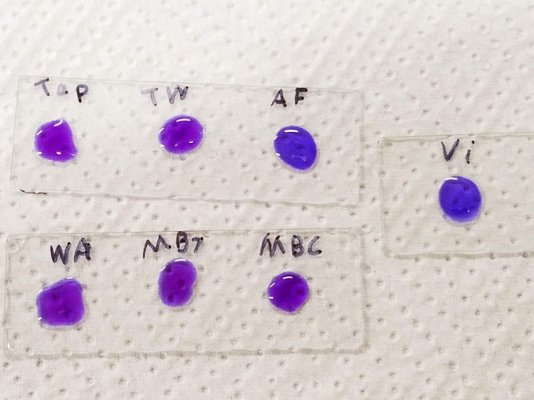
Top slide: tap water, tank water, AlgaeFix marine.
Bottom slide: Waste Away, MicroBacter 7, MicroBacter Clean
Right slide: Vibrant
The bromophenol blue kept its normal purple appearance for all except Algaefix and Vibrant, which caused it to turn blue.
(vibrant, algaefix, and their dried residues are slightly acidic - so could not be causing the BPB to shift to blue by high pH)
That AlgaeFix showed the response predicted in the paper is exactly as expected - the active ingredient is a known quaternary ammonia compound (read more about the algaefix ingredient here).
That Vibrant gave the same response is a surprise (except maybe to @jda ). It seems that looking to the chemistry of the media may be a much simpler and more direct route to explaining Vibrant's algae-killing properties than trying to do elaborate bacterial tests.
@jeffww and @Dan_P helped propose and eliminate a number of potential reasons for a misleading result here, but no dice so far.
This might be worth trying: https://pubmed.ncbi.nlm.nih.gov/33191523/ should(conjecture) detect if quat like algaefix is in the mixture.
from the paper, "This study investigates the utility of bromophenol blue (BPB) as a safe, rapid and user-friendly indicator to detect in situ residual QACs [quaternary ammonium compounds] dried on hard, non-porous surfaces, as well a means to assess their antimicrobial efficacy. At pH 7, BPB has a purple colour which turns blue upon its complexation with QACs..."
So I dried drops of a few different things on glass slides and then applied a drop of bromophenol blue to the residue of each.

Top slide: tap water, tank water, AlgaeFix marine.
Bottom slide: Waste Away, MicroBacter 7, MicroBacter Clean
Right slide: Vibrant
The bromophenol blue kept its normal purple appearance for all except Algaefix and Vibrant, which caused it to turn blue.
(vibrant, algaefix, and their dried residues are slightly acidic - so could not be causing the BPB to shift to blue by high pH)
That AlgaeFix showed the response predicted in the paper is exactly as expected - the active ingredient is a known quaternary ammonia compound (read more about the algaefix ingredient here).
That Vibrant gave the same response is a surprise (except maybe to @jda ). It seems that looking to the chemistry of the media may be a much simpler and more direct route to explaining Vibrant's algae-killing properties than trying to do elaborate bacterial tests.
@jeffww and @Dan_P helped propose and eliminate a number of potential reasons for a misleading result here, but no dice so far.
- Joined
- Jul 18, 2019
- Messages
- 330
- Reaction score
- 542
Thanks to @taricha for having the inertia to try this! While not absolute confirmation, I think we can be relatively certain there is some ingredient, not listed on the label, that is able to chelate bromophenol blue and this might be some type of strong chelating cation akin to quarternary amine.
The types of these compounds that can do this seem pretty few in my hands and quaternary ammonium like algaefix is most likely. I paneled some 7 materials in our lab that may have similar properties to quaternary ammonium or could be found in a product like this. I especially sought things containing nitrogen in varying protonation states or common cations. These include:
100mM Calcium, 100mM Guanidinium Chloride, 1%(v/v) Branched Polyethylenimine, quaternary amine water bath cleaner, LB broth, 100mM ammonium bicarbonate, L-arginine (posses primary amine and guanidine group). Of these, only branched PEI and quat based cleaner gave positive results. PEI was a little surprising since it is comprised soley of primary/secondary/tertiary amines. However, we use it in lab to chelate anionic DNA and it is used to fix anionic dyes to fabrics industrially, so this is understandable.
Although I don't have the particular gumption to run a full MS analysis on the product, I'd be intrigued if spotting algaefix next to vibrant on a tlc plate and spraying with bromophenol blue would reveal compounds of similar migration...hmmm.
The types of these compounds that can do this seem pretty few in my hands and quaternary ammonium like algaefix is most likely. I paneled some 7 materials in our lab that may have similar properties to quaternary ammonium or could be found in a product like this. I especially sought things containing nitrogen in varying protonation states or common cations. These include:
100mM Calcium, 100mM Guanidinium Chloride, 1%(v/v) Branched Polyethylenimine, quaternary amine water bath cleaner, LB broth, 100mM ammonium bicarbonate, L-arginine (posses primary amine and guanidine group). Of these, only branched PEI and quat based cleaner gave positive results. PEI was a little surprising since it is comprised soley of primary/secondary/tertiary amines. However, we use it in lab to chelate anionic DNA and it is used to fix anionic dyes to fabrics industrially, so this is understandable.
Although I don't have the particular gumption to run a full MS analysis on the product, I'd be intrigued if spotting algaefix next to vibrant on a tlc plate and spraying with bromophenol blue would reveal compounds of similar migration...hmmm.
Fascinating stuff. While I don't have the technical skills to contribute, I really appreciate others going the extra mile to try and provide a clearer picture on this!
- Joined
- Jul 18, 2019
- Messages
- 330
- Reaction score
- 542
Fascinating stuff. While I don't have the technical skills to contribute, I really appreciate others going the extra mile to try and provide a clearer picture on this!
Yep, seems likely that vibrant contains quaternary ammonium which are common biocides and is the active reagent in AlgaeFix, although algaefix is around 5 dollars a bottle and actually is up front with its contents. Quats are common disinfectants think odoban, contact solution, pool cleaner, clorox wipes etc.
- Joined
- Oct 24, 2016
- Messages
- 1,547
- Reaction score
- 2,377
Not sure how widely known this is. With the right manipulation bacteria can produce polymides or strait poly, aspartic acid. This is most easily achieved with certain bacillus and Cyanobacteria species. When they are “forced” to produce poly, this adds a function for aquarium use that is very good at removing flocculants and is very good for clearing water 
- Joined
- Dec 28, 2016
- Messages
- 22,709
- Reaction score
- 21,894
This is interesting! The usual use for Bromphenol blue is as a pH indicator. The starting color is yellow - the and end color is blue. It becomes blue at 4.6. - pH 7 - and then purple as the pH rises. Multiple things can affect the color change - try adding an alkali solution - I wonder if as a control solution - if you added various alkali - with pH 8,9,10, etc if you could see the same change. I guess it all depends on when you see the 'color change' - if any as you add alakali. For example if its at pH 12 - its unlikely to be living bacteria in the bottle. If its at pH 9 thats a different story.Thanks to @taricha for having the inertia to try this! While not absolute confirmation, I think we can be relatively certain there is some ingredient, not listed on the label, that is able to chelate bromophenol blue and this might be some type of strong chelating cation akin to quarternary amine.
The types of these compounds that can do this seem pretty few in my hands and quaternary ammonium like algaefix is most likely. I paneled some 7 materials in our lab that may have similar properties to quaternary ammonium or could be found in a product like this. I especially sought things containing nitrogen in varying protonation states or common cations. These include:
100mM Calcium, 100mM Guanidinium Chloride, 1%(v/v) Branched Polyethylenimine, quaternary amine water bath cleaner, LB broth, 100mM ammonium bicarbonate, L-arginine (posses primary amine and guanidine group). Of these, only branched PEI and quat based cleaner gave positive results. PEI was a little surprising since it is comprised soley of primary/secondary/tertiary amines. However, we use it in lab to chelate anionic DNA and it is used to fix anionic dyes to fabrics industrially, so this is understandable.
Although I don't have the particular gumption to run a full MS analysis on the product, I'd be intrigued if spotting algaefix next to vibrant on a tlc plate and spraying with bromophenol blue would reveal compounds of similar migration...hmmm.
By the way - here is the 'method' for doing the test - as compared to doing it on a slide which may be helpful confirmation that what you're seeing MAY be QAC's:
"
Bromophenol blue test22
Bromophenol blue solution: Add 20 ml of 0.1% bromophenol blue in 96% ethanol to a mixture of 75 ml 0.2 N sodium acetate and 925 ml 0.2 N acetic acid. Adjust the pH of the solution to 3.6–3.9.Test: Add 2-5 drops of a neutralized sample solution to 10 ml of bromophenol blue solution. Shake well and observe the color of the mixture. If a blue color is shown, the existence of a cationic surfactant is confirmed.
Alternatively, add one drop of 5% sample solution to a mixture of 5 ml chloroform, 5 ml 0.1% bromophenol blue dilute ethanol solution and 1 ml 6 N HCl. Shake well and observe the color of the chloroform layer. If a yellow color appears, there exists a cationic surfactant in the sample."
- Joined
- Jul 18, 2019
- Messages
- 330
- Reaction score
- 542
Hi! I think I have polyglutamic acid, a similar anionic amino acid polymer. I can verify if this material can produce a color change. I highly doubt they will since both polyQ and polyD are both negatively charged, chemically similar and are unlikely to chelate the negatively charged dye. Heck, I'll even check some synthetic DNA today.Not sure how widely known this is. With the right manipulation bacteria can produce polymides or strait poly, aspartic acid. This is most easily achieved with certain bacillus and Cyanobacteria species. When they are “forced” to produce poly, this adds a function for aquarium use that is very good at removing flocculants and is very good for clearing water
Nextly, I doubt there are cyanobacteria are in vibrant since the solution is clear and are not colored as cyanobacteria tend to be. In terms of bacillus, I guess they could be in there but the inactive spores of bacillus wouldn't be undergoing metabolism and probably wouldn't be producing these polyAAs, furthermore it wouldn't explain the algaecidal properties of vibrant since flocculants already exist for aquariums and they are not algaecide products. Although, to my understanding they could aid in the clearance of green water.
The pH of the BPB solution is controlled with excess phosphate buffer such that the pH contribution of any added analytes does not shift the color. Furthermore, the BPB colorshift to basic pH does not occur. @taricha has performed this test. Finally, the pH of both algaefix and vibrant are slightly acidic not basic.This is interesting! The usual use for Bromphenol blue is as a pH indicator. The starting color is yellow - the and end color is blue. It becomes blue at 4.6. - pH 7 - and then purple as the pH rises. Multiple things can affect the color change - try adding an alkali solution - I wonder if as a control solution - if you added various alkali - with pH 8,9,10, etc if you could see the same change. I guess it all depends on when you see the 'color change' - if any as you add alakali. For example if its at pH 12 - its unlikely to be living bacteria in the bottle. If its at pH 9 thats a different story.
By the way - here is the 'method' for doing the test - as compared to doing it on a slide which may be helpful confirmation that what you're seeing MAY be QAC's:
"
Bromophenol blue test22
Bromophenol blue solution: Add 20 ml of 0.1% bromophenol blue in 96% ethanol to a mixture of 75 ml 0.2 N sodium acetate and 925 ml 0.2 N acetic acid. Adjust the pH of the solution to 3.6–3.9.
Test: Add 2-5 drops of a neutralized sample solution to 10 ml of bromophenol blue solution. Shake well and observe the color of the mixture. If a blue color is shown, the existence of a cationic surfactant is confirmed.
Alternatively, add one drop of 5% sample solution to a mixture of 5 ml chloroform, 5 ml 0.1% bromophenol blue dilute ethanol solution and 1 ml 6 N HCl. Shake well and observe the color of the chloroform layer. If a yellow color appears, there exists a cationic surfactant in the sample."
In regards to your method, the method we both used was adapted from literature. But there are multiple ways of employing bromophenol blue to the detection of quaternary amines with or without chloroform extraction of the final complex. Basically as long as you add excess buffer and apply the solution to QAC, a color change occurs. No extraction is needed unless downstream analysis of the QAC is desired. OR if the analyte is in an unfriendly solution like milk. Considering that vibrant is clear as water. I don't think this is the case. Here are some literature (you can PM me if you'd like access):
Poly-γ-glutamate-based Materials for Multiple Infection Prophylaxis Possessing Versatile Coating Performance
Poly-γ-glutamate (PGA) possesses a nylon-like backbone and polyacrylate-like carboxyl groups, and shows an extraordinary solubility in water. In this study, the effective synthesis and structural analysis of some water-insoluble PGA ion-complexes (PGAICs) using cationic surfactants...

Colorimetric detection of residual quaternary ammonium compounds on dry surfaces and prediction of antimicrobial activity using bromophenol blue - PubMed
Controlling and monitoring the residual activity of quaternary ammonium compounds (QACs) are critical for maintaining safe yet effective levels of these agents in the environment. This study investigates the utility of bromophenol blue (BPB) as a safe, rapid and user-friendly indicator to detect...
Towards an antimicrobial ‘microglove’ - Scientific Reports
A large proportion of hospital-related infections are acquired and spread due to the direct contacts between patients and healthcare workers. Accordingly, proper infection prevention measures and especially hand hygiene, are key to limit the spread of infections in nosocomial settings. However...
In the end. Vibrant likely contains quaternary ammonia or some other unlisted, highly charged compound capable of binding anionic dyes. Either way, its activity probably has nothing to do with anything listed on the label, in my opinion
Similar threads
- Replies
- 1
- Views
- 77
- Replies
- 4
- Views
- 421
- Replies
- 62
- Views
- 1,404