I have previously run the BRS reactor with GFO for the past 2 years, my PO4 numbers have been struggling to drop below 0.15-0.2 ppm, despite changing the media once a week. With the reactor off my return pump I could only get 10 GPH (neptune flow meter) and it would channel the media. So I moved up to a Avast spyglass which fluidizes the media (now HC GFO) much better and I can control the flow upto ~150 gph without issue.
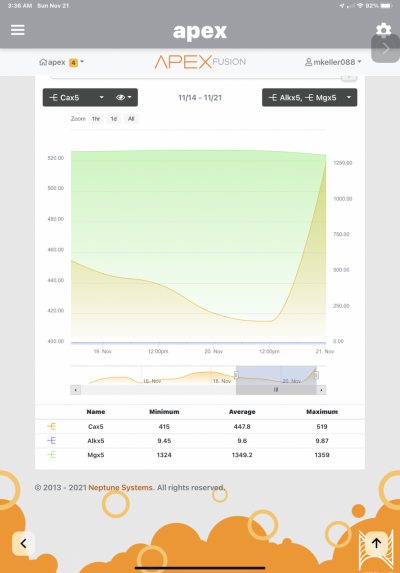
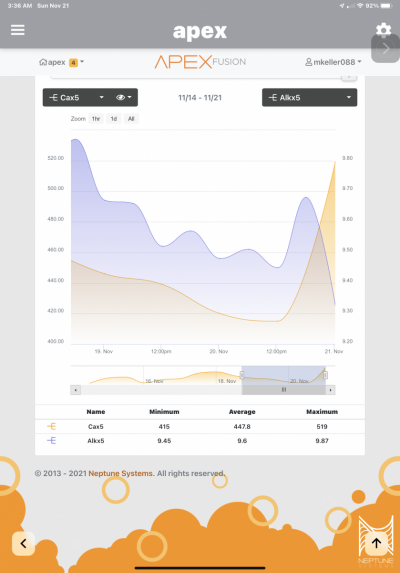
Now for the interesting part, over the last week of setting up this reactor my Calcium has risen from 450 to 630 via trident, and it increases daily. For the last week I haven't dosed calcium and even turned off my AWC. My salt mix, Tropic marine pro, is currently 440 via Red sea test and not know to be a high calcium salt.
I think this is attributed to the improved PO4 removal that is liberating Calcium from calcium phosphate precipitate in the tank from previous ineffective removal from previous method. And since its in equilibrium with free PO4 and Ca, this would explain the rise.
I am looking to verify if this logic makes sense and when should I worry about calcium levels, at this rate they are rising ~20 ppm every 12 hrs, I started AWC back again since replacing water with lower calcium NSW should work in my favor. Any other ideas to help lower or at least stabilize the calcium levels?
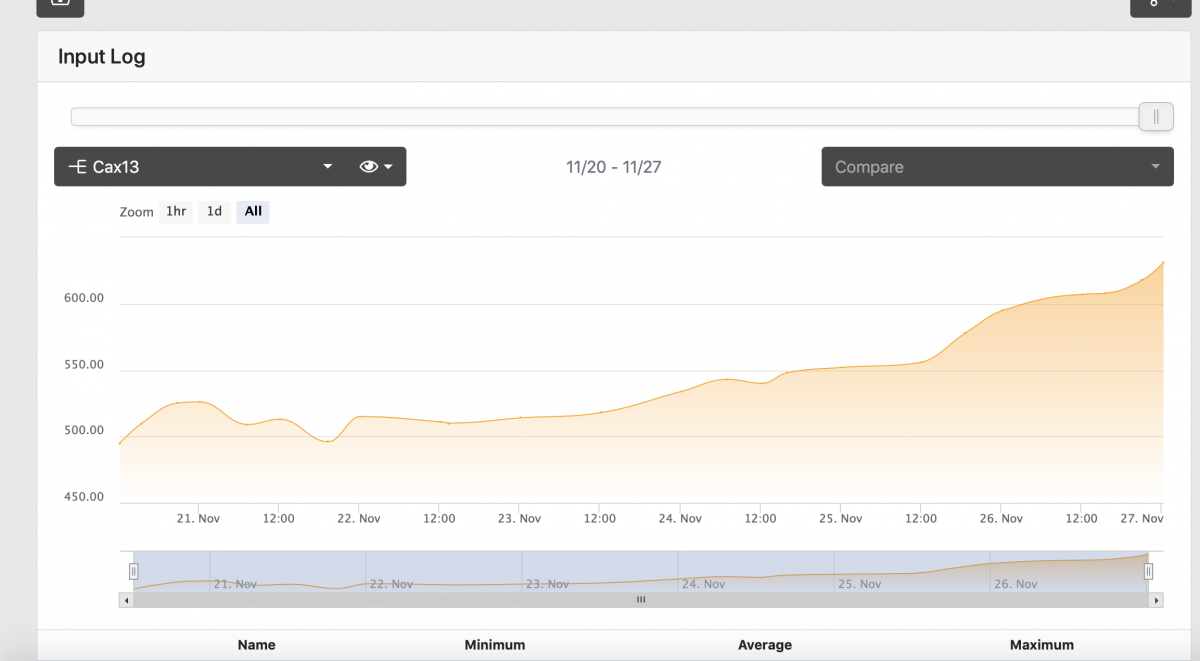
Now for the interesting part, over the last week of setting up this reactor my Calcium has risen from 450 to 630 via trident, and it increases daily. For the last week I haven't dosed calcium and even turned off my AWC. My salt mix, Tropic marine pro, is currently 440 via Red sea test and not know to be a high calcium salt.
I think this is attributed to the improved PO4 removal that is liberating Calcium from calcium phosphate precipitate in the tank from previous ineffective removal from previous method. And since its in equilibrium with free PO4 and Ca, this would explain the rise.
I am looking to verify if this logic makes sense and when should I worry about calcium levels, at this rate they are rising ~20 ppm every 12 hrs, I started AWC back again since replacing water with lower calcium NSW should work in my favor. Any other ideas to help lower or at least stabilize the calcium levels?
Last edited: