I got the opportunity to be one of the first tanks to try the Triton Water Test back in July.
I was pretty surprised by my results. Since I don't do water changes, and I don't dose anything regularly (or accurately). My dosing consists of my Calcium RX and Kalkwasser for Topoff.
These are the results from July 25, 2014
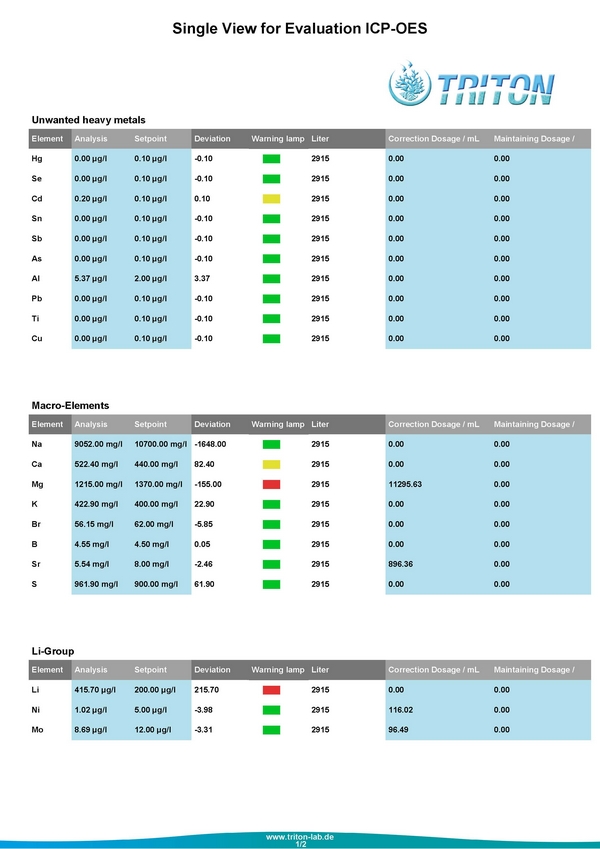
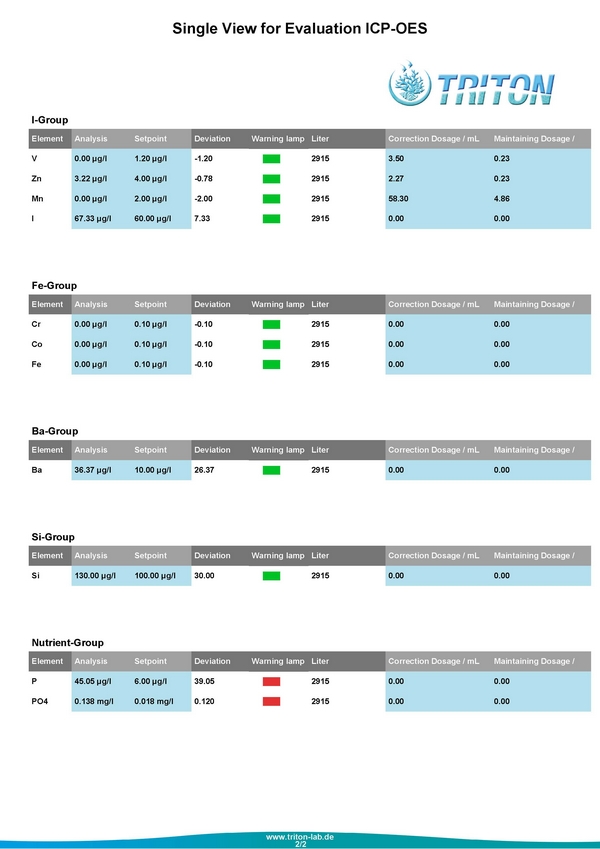
I wasn't surprised by my Phosphate levels, but thought that was one of the things I could easily resolve. The first step was I pulled out my little Hanna Pocket Colorimeter the $50 toy, and was happy to see it was within 2 1/100ths of the Triton value. I pulled out my reliable Deltec 509 Fluidized Reactor and put in maybe 3 cups worth of media.
After a week the phosphate levels had dropped by half. In August and Sept I stayed on top of replacing the GFO every 3-4 weeks. However, for the past 6 weeks I have been dealing with an ill parent and I have left the tank untouched, other than the Alk issues from the Calc Rx. I haven't had time for any maintenance.
Last week I sent in another water sample to see how things were looking, and these are the second round results
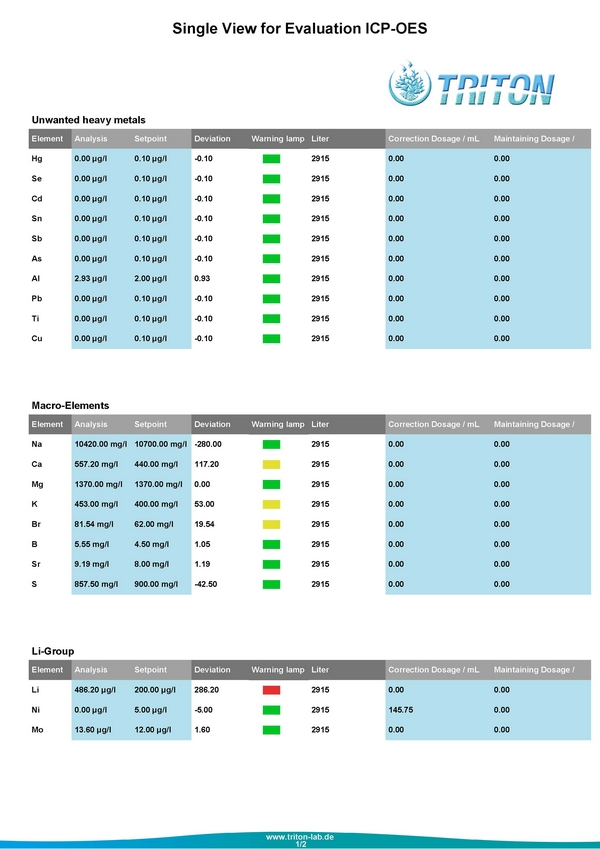
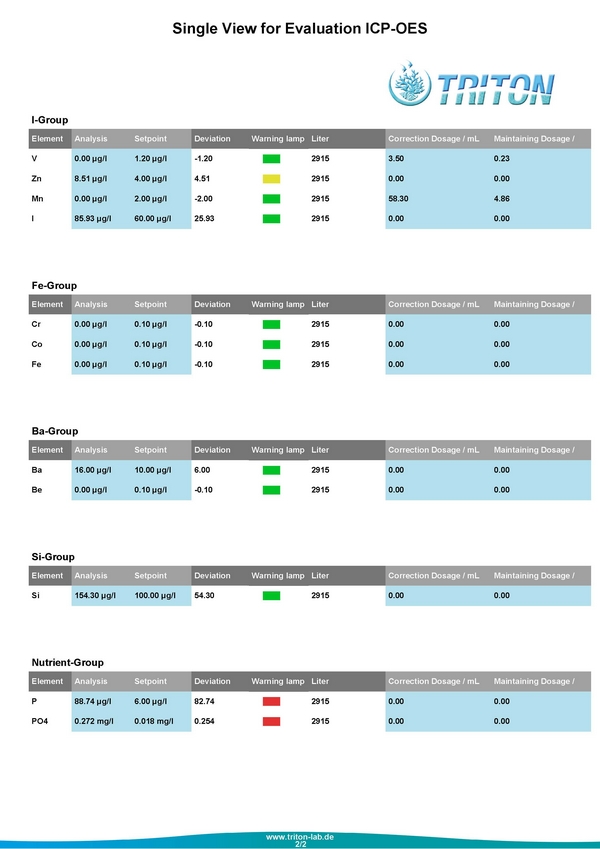
Tonight when I get home, I will definitely be cleaning out the Deltec Rx and putting in a fresh batch of GFO and work on getting the Phosphates back down. At the moment, even with these high phosphate levels the corals in the tank look really good. The only coral that sticks out as not looking as good as it could is my Suharsanoi. It's color lightened dramatically when the Alk was bouncing all over the place, and it has darkened back up yet.
Dave B
I was pretty surprised by my results. Since I don't do water changes, and I don't dose anything regularly (or accurately). My dosing consists of my Calcium RX and Kalkwasser for Topoff.
These are the results from July 25, 2014


I wasn't surprised by my Phosphate levels, but thought that was one of the things I could easily resolve. The first step was I pulled out my little Hanna Pocket Colorimeter the $50 toy, and was happy to see it was within 2 1/100ths of the Triton value. I pulled out my reliable Deltec 509 Fluidized Reactor and put in maybe 3 cups worth of media.
After a week the phosphate levels had dropped by half. In August and Sept I stayed on top of replacing the GFO every 3-4 weeks. However, for the past 6 weeks I have been dealing with an ill parent and I have left the tank untouched, other than the Alk issues from the Calc Rx. I haven't had time for any maintenance.
Last week I sent in another water sample to see how things were looking, and these are the second round results


Tonight when I get home, I will definitely be cleaning out the Deltec Rx and putting in a fresh batch of GFO and work on getting the Phosphates back down. At the moment, even with these high phosphate levels the corals in the tank look really good. The only coral that sticks out as not looking as good as it could is my Suharsanoi. It's color lightened dramatically when the Alk was bouncing all over the place, and it has darkened back up yet.
Dave B









