Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,311
- Reaction score
- 63,658
The molecule shown below in a 3D diagram is shown in two different forms, both of which are present in normal reef aquaria.
What is the most likely identity of this molecule?
A. Ammonia/ammonium
B. Borate/Boric Acid
C. two forms of phosphate
D. Nitrate and nitrite
E. Bicarbonate/carbonate
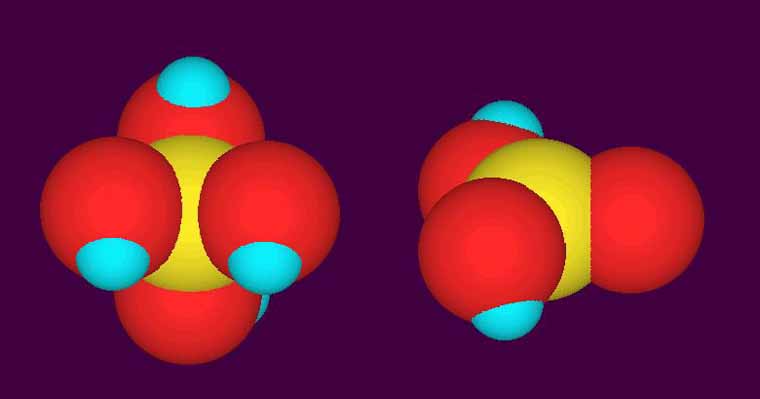
Good luck!
.
What is the most likely identity of this molecule?
A. Ammonia/ammonium
B. Borate/Boric Acid
C. two forms of phosphate
D. Nitrate and nitrite
E. Bicarbonate/carbonate
Good luck!
.
Last edited:


















