Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,288
- Reaction score
- 63,633
Reef Chemistry Question of the Day #78
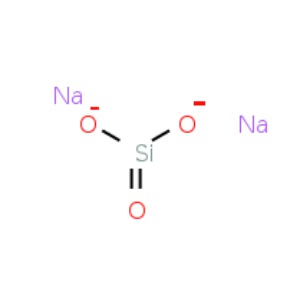
One of the few things that I dose to my aquarium is sodium silicate solution to help provide silica to sponges and diatoms.
Which of the following is not a consequence of this dosing?
A. There is local precipitation of sodium carbonate at the point of addition
B. There is local precipitation of magnesium hydroxide at the point of addition
C. The pH rises
D. The silicic acid level rises
E. The alkalinity rises
F. The silicate level rises
Feel free to pick more than one if you believe it appropriate.
Good luck!
.
One of the few things that I dose to my aquarium is sodium silicate solution to help provide silica to sponges and diatoms.
Which of the following is not a consequence of this dosing?
A. There is local precipitation of sodium carbonate at the point of addition
B. There is local precipitation of magnesium hydroxide at the point of addition
C. The pH rises
D. The silicic acid level rises
E. The alkalinity rises
F. The silicate level rises
Feel free to pick more than one if you believe it appropriate.
Good luck!
.