I will mix up some more tomorrow. I'm out of RO/DI water.Please mix up a batch of saltwater and aerate a cup of it outside with an aitstone for 1hr.
Report back pH and Alk
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
RO/DI water plus Red Sea Salt = 7.1 pH?
- Thread starter whybenormal
- Start date
- Tagged users None
I didn't check the alkalinity, only the pH, but that water had already been dumped out before I saw your suggestion.did you check the alkalinity after mixing? I suggested that earlier.
If you aerate it, does it rise?
I will make more water tomorrow and try it again and try aerating it as well.
I'm not sure about testing before the DI resin. I know that the pH at the tap and after it makes RO/DI are the same. 7.1.What about testing the pH before the DI resin?
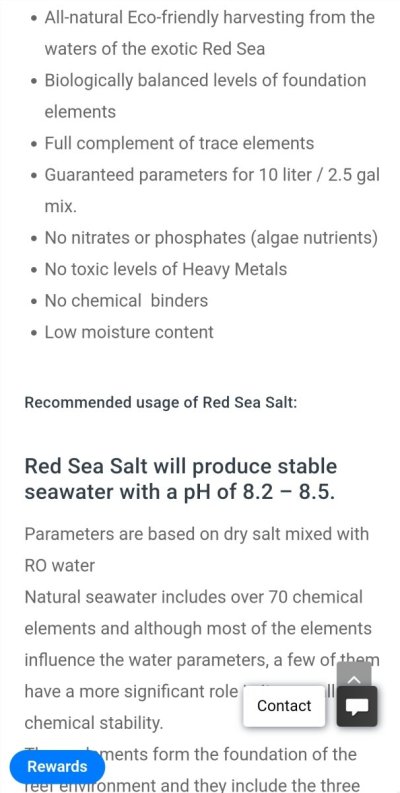
Here is just one place I read the statement about pHHow does Red Sea stat the PH their salt mixes to? It depends on outside variables. Could you possibly be referring to alkalinity? Sorry I'm a little confused...ignore me if you wish
And here on their website they tell you the alkalinity also.
Wanting something with good stability is my biggest reason for switching.
We just got it in September. All in all it has probably had about 150-200 gallons produced.May or may not be the cause of your pH issue, but if tap TDS is 109, the TDS after the membrane should be no higher than 5 or 6 (depending on the specific membrane you use) assuming the correct water pressure. Once the water is filtered through DI reason, TDS should drop to close to 0.
How recently have you changed the sediment and carbon filters? If they are relatively new, I suspect you need to change the membrane and/or it's not working correctly.
We haven't changed any filters yet.
I'm not quite sure when you say the TDS after the membrane should be no higher than 5-6 (it is 6) and once it is filtered though DI it should drop close to 0.
After which membrane should it be 5-6?
Right, KH, not PH. Red Sea (like all salt brands) state their KH which is their alkalinity level, this is not PH. I wonder if some terms are simply being mixed up here.
I can hardly do the science justice so I'll post an article from forum owner himself which breaks it down quite well for us
pH And The Reef Aquarium
For many aquarists, pH is not something that they have much experience with aside from their aquarium. For many, pH is almost a black box measurement: something to be considered, but whose physical meaning makes little sense to them. This...
 www.reef2reef.com
www.reef2reef.com
The RO membrane. It goes sediment -> carbon -> ro membrane -> DIWe just got it in September. All in all it has probably had about 150-200 gallons produced.
We haven't changed any filters yet.
I'm not quite sure when you say the TDS after the membrane should be no higher than 5-6 (it is 6) and once it is filtered though DI it should drop close to 0.
After which membrane should it be 5-6?
Here is what they are pointing out.Right, KH, not PH. Red Sea (like all salt brands) state their KH which is their alkalinity level, this is not PH. I wonder if some terms are simply being mixed up here.
I can hardly do the science justice so I'll post an article from forum owner himself which breaks it down quite well for us

pH And The Reef Aquarium
For many aquarists, pH is not something that they have much experience with aside from their aquarium. For many, pH is almost a black box measurement: something to be considered, but whose physical meaning makes little sense to them. This...www.reef2reef.com
Interesting. I don't use RS fwiw. How can they state that? Do I not understand PH? Seachem has 8.3 buffer and AquaVitro sells 8.4. I figured these were just bs marketing ploys.... They CAN get you to that PH but staying there is not just hit n run....
To test the TDS after the membrane (and before the DI), you'll need to unhook the tubing that goes from your membrane to the first (if you have more than one) DI canister. Unhook it where it enters the DI canister. Then run the unit for a few minutes and then take a sample and test TDS.The RO membrane. It goes sediment -> carbon -> ro membrane -> DI
Depending on where everything is set up, you'll probably need to hold a bucket to catch the water coming out of the tube you detached... Again, let it run for a few minutes and then catch some of the water in a clean glass, etc, and test that sample for your TDS level. (The reason to run it for a bit first is to account for anything that flushes out of the membrane when it's started up... Not anything to worry about when you're filtering water, but you want the sample to be as 'pure' as possible)
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,122
- Reaction score
- 63,463
Interesting. I don't use RS fwiw. How can they state that? Do I not understand PH? Seachem has 8.3 buffer and AquaVitro sells 8.4. I figured these were just bs marketing ploys.... They CAN get you to that PH but staying there is not just hit n run....
What salt mixes can claim is what the pH is when mixed with pure water and no aeration/CO2 exchange (which may be correct), or the pH after equilibration with normal air (which depends on air CO2 levels and is easily wrong and may be much lower than claimed).
The buffers are marketing ploys.
Providing these numbers on an outside aerated cup of mixed ro/di seawater will answer both mine and @randyquestions...or lead to a more intriguing mystery.I will mix up some more tomorrow. I'm out of RO/DI water.
This is I was thinking as well just from what I've come to understand. In certain specific environment/scenario the claim can be made I supposeWhat salt mixes can claim is what the pH is when mixed with pure water and no aeration/CO2 exchange (which may be correct), or the pH after equilibration with normal air (which depends on air CO2 levels and is easily wrong and may be much lower than claimed).
The buffers are marketing ploys.
I like very much such a mysterious cases 
But I think the answer is quite simple. There is no way if the salt is OK, to have no change of pH after the salt is dissolved. Doesn't matter if RO/DI system is working well or not. Even if you are using tap water and if the salt mix is good you will have about 8-8,3 pH after the salt is dissolved. The tap water to be allowed to be used for drinking should have pH around 7 .
.
From what you are saying is quite clear the problem is not in the pH measurement.
The most obvious reason then is your salt mix. All the artificial dry salt mixes contained (aside of sodium chloride) sodium carbonate/ bicarbonate and calcium chloride in dry form. The main purpose of the carbonate and bicarbonate is to ensure pH and Alkalinity. The problem is they will react with the calcium chloride in the mix if there is even small amount of water/moisture in the mix. That is why is extremely important salt mixes to be kept in very dry environment. In presence of water, carbonates will react with calcium chloride forming sodium chloride and calcium carbonate. The process is quite fast and may lead to total consumption of the sodium carbonates in the mix. As a final result the salt mix will have limited capacity to increase the pH and Alk (and also calcium because the calcium carbonate is almost insoluble in water).
Just check your salt if doesn't look like fine dry sand but looks humid or is in big chucks, then the reason is salt was kept unsealed in humid environment. But the most simple test is to check alkalinity, I bet it will be very very low.
And this is not about the brand of the salt, this will happened to every dry salt mix which is kept unsealed in humid environment (in the store or in your home). The quality of the Red Sea salts is actually very good IMO - Im using their salts more than 15 years with quite good results, and I'm very happy they have two versions with 12 and 8 kH
But I think the answer is quite simple. There is no way if the salt is OK, to have no change of pH after the salt is dissolved. Doesn't matter if RO/DI system is working well or not. Even if you are using tap water and if the salt mix is good you will have about 8-8,3 pH after the salt is dissolved. The tap water to be allowed to be used for drinking should have pH around 7
From what you are saying is quite clear the problem is not in the pH measurement.
The most obvious reason then is your salt mix. All the artificial dry salt mixes contained (aside of sodium chloride) sodium carbonate/ bicarbonate and calcium chloride in dry form. The main purpose of the carbonate and bicarbonate is to ensure pH and Alkalinity. The problem is they will react with the calcium chloride in the mix if there is even small amount of water/moisture in the mix. That is why is extremely important salt mixes to be kept in very dry environment. In presence of water, carbonates will react with calcium chloride forming sodium chloride and calcium carbonate. The process is quite fast and may lead to total consumption of the sodium carbonates in the mix. As a final result the salt mix will have limited capacity to increase the pH and Alk (and also calcium because the calcium carbonate is almost insoluble in water).
Just check your salt if doesn't look like fine dry sand but looks humid or is in big chucks, then the reason is salt was kept unsealed in humid environment. But the most simple test is to check alkalinity, I bet it will be very very low.
And this is not about the brand of the salt, this will happened to every dry salt mix which is kept unsealed in humid environment (in the store or in your home). The quality of the Red Sea salts is actually very good IMO - Im using their salts more than 15 years with quite good results, and I'm very happy they have two versions with 12 and 8 kH
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,122
- Reaction score
- 63,463
I like very much such a mysterious cases
But I think the answer is quite simple. There is no way if the salt is OK, to have no change of pH after the salt is dissolved. Doesn't matter if RO/DI system is working well or not. Even if you are using tap water and if the salt mix is good you will have about 8-8,3 pH after the salt is dissolved. The tap water to be allowed to be used for drinking should have pH around 7.
From what you are saying is quite clear the problem is not in the pH measurement.
The most obvious reason then is your salt mix. All the artificial dry salt mixes contained (aside of sodium chloride) sodium carbonate/ bicarbonate and calcium chloride in dry form. The main purpose of the carbonate and bicarbonate is to ensure pH and Alkalinity. The problem is they will react with the calcium chloride in the mix if there is even small amount of water/moisture in the mix. That is why is extremely important salt mixes to be kept in very dry environment. In presence of water, carbonates will react with calcium chloride forming sodium chloride and calcium carbonate. The process is quite fast and may lead to total consumption of the sodium carbonates in the mix. As a final result the salt mix will have limited capacity to increase the pH and Alk (and also calcium because the calcium carbonate is almost insoluble in water).
Just check your salt if doesn't look like fine dry sand but looks humid or is in big chucks, then the reason is salt was kept unsealed in humid environment. But the most simple test is to check alkalinity, I bet it will be very very low.
And this is not about the brand of the salt, this will happened to every dry salt mix which is kept unsealed in humid environment (in the store or in your home). The quality of the Red Sea salts is actually very good IMO - Im using their salts more than 15 years with quite good results, and I'm very happy they have two versions with 12 and 8 kH
I personally still think the pH 7.1 was likely measurement error and the 7.1 won’t repeat with a new batch.
it cannot be a bad batch of salt if making the salt with distilled water gave the expected pH.
Thank you for posting that. I was just going to do that when I saw you had.
Ahh my bad, I missed that part testing with distilled / bottled water. Then agree on measurement error. I can't imagine tap water could be that potent 7 pH buffer solutionit cannot be a bad batch of salt if making the salt with distilled water gave the expected pH.
When you start out is the RO/DI Water Cold? I found out with Reef Crystals, and Cold Water, I don’t get a good mix, by dumping all the Salt in at once. I go with half the Salt, add the Heater. After the Salt is completely dissolve, and water is clear. I dose 1/4 the Salt, then slowly bring it up to 34.5ppt. BTW, initially I use a Danner 2 pump, in a 30 or 40 Gallon Brute.
If ph locked in to what was posted by the manufacturer there would be no market for ph additives/mechanisms. I feel like this is an unfair statement made by them. Ph fluctuates throughout the day. An ionic balance that promotes desired ph levels is all they can say imo. I urge you to not pay too much attention to that really. Good luckThank you for posting that. I was just going to do that when I saw you had.
Providing these numbers on an outside aerated cup of mixed ro/di seawater will answer both mine and @randyquestions...or lead to a more intriguing mystery.
Any update here?
Similar threads
- Replies
- 6
- Views
- 111
- Replies
- 12
- Views
- 405
- Replies
- 27
- Views
- 340
- Replies
- 10
- Views
- 290

















