Well this will be my third trip down this “Rabbit Hole”…One would think I should have learned my lesson, but Noooooo! I jumped back in with both feet! If you are brave enough come with me on my third journey into “Wonderland” then read on!!

In my last post in July of last year I presented the case that samples sent for ICP analysis were not completely stable and that during the storage time before testing there was a change (reduction) in the measurable level of Phosphorous and thus the reported PO4 values from the ICP test results were consistently lower than my measurements using the Hanna Tester. This was demonstrated by several experiments including sampling to an ICP vendor and replicating the results. Through additional experiments it was found that a 7ppm add of bleach to the samples would stabilize the samples and keep the measurable amount phosphorous from being reduced. This was also demonstrated via several experiments as well as ICP test results. If you are interested in the details here is a link to the post.

 www.reef2reef.com
www.reef2reef.com
At the end of the post I mentioned that based on the results of this work there were additional questions to be explored as well as some proposed questions from the comments to the post. This trip to “Wonderland” attempts to address some of these.
Questions and Issues Explored
Thanks to @Dan_P and @taricha for their help. As always their contributions were of great value!
IODINE---IRON---PHOSPHORUS STORAGE IMPACT EXPERIMENT
EXPERIMENTAL LAYOUT
A large sample of tank water was collected. The sample was then filtered through a 20 micron filter to remove any suspended material. (See Note on Nitric Acid Evaluation)* Part of the sample was separated out and “spiked” with Iron to raise the level to a measurable level. Measurements were made on this initial sample for Iron, Iodine and Phosphorus. There were 5 measurements made on each and an average taken to represent the “INITIAL MEASUREMENT” value.
The two samples (Iron Spiked & Un-Spiked) were each separated into 3 sample sets. One sample set would be the baseline (NO PRESERVATIVE): The second set was treated with 7ppm Cl: The third set was treated with 300 ppm Nitric Acid (See Note on Nitric Acid Evaluation)*. That would be 6 sample sets altogether. The six sets were then placed in 15 mL sample tubes and place in “storage” at room temperature 70⁰ F (21 C) The sample flow chart below will better explain this…I think
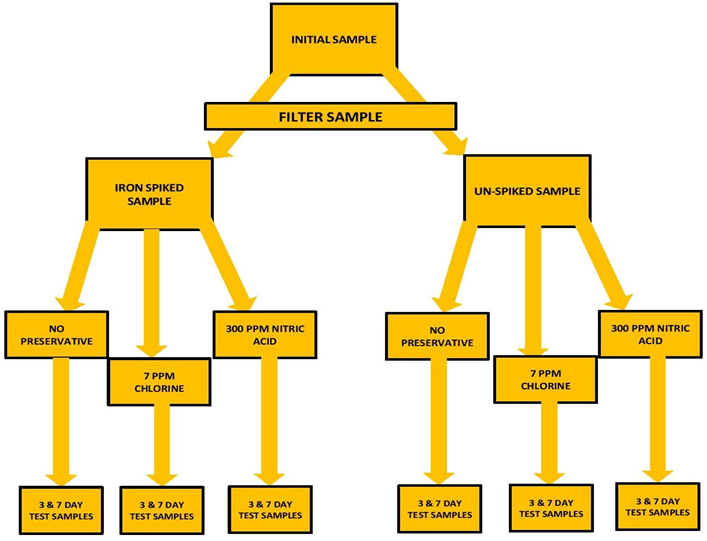
At the end of 3 days of storage samples were removed and measured for: Iron, Iodine, and Phosphorus. Three measurements were taken for each of the test sample sets. That would be 9 measurements for each of the Elements…3 for No Preservative…3 for the Chlorine…and 3 for the Nitric Acid…27 sample measurements total for the 3 day storage samples. The very same protocol was done for day 7.
TEST METHODS
Iron was tested using the Hanna HI-746 Low Range Iron Checker. It has a reported accuracy of ±20 ppb ± 5% of the reading.
Iodine was tested by a method I developed using the Red Sea Chemistry and HI-707 Low Range Nitrite Checker. It has an error of ± 5% of the reading (Here is a link to the method. https://www.reef2reef.com/threads/using-hanna-checker-hi-707-to-test-for-iodine.669022/ )
Phosphorus was tested using the Hanna HI-736 ULR Phosphorus Checker. It has a reported accuracy of ±5ppb ± 5% of the reading.
The error ranges are reflected in the error bars included on the charts.
The results can be seen in TABLE 1 CHARTS 1-6.
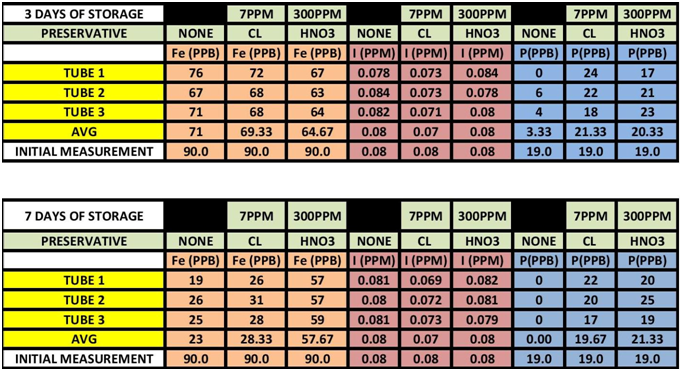
TABLE 1
IRON
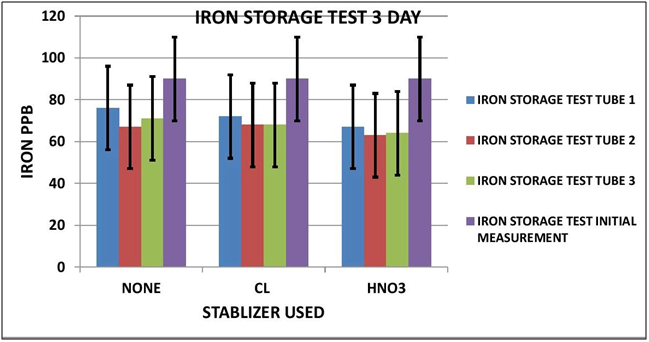
CHART 1
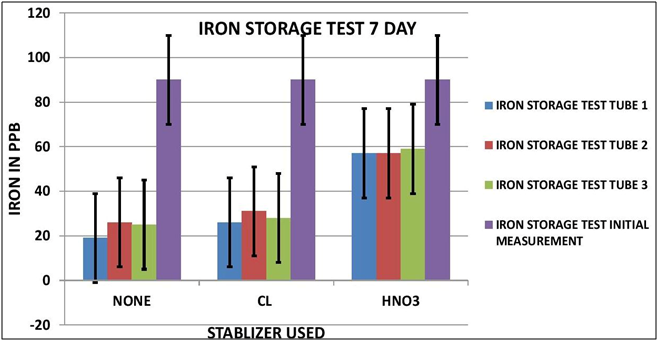
CHART 2
IRON OBSERVATIONS
The levels of Iron in natural seawater are very low, .006 ppb. This level is well below where we as hobbyist would be able to measure with any level of confidence. The HI-764 has a stated accuracy of ± 20 ppb which is many times the natural level. Also Iron is very transient in the aquarium. It is used up very quickly so even if you are dosing iron, it will be quickly depleted in the tank. So you might be asking yourself “Why did you include Iron in this experiment?” I would say mostly curiosity! I wanted to see if elevated levels of iron (> 90 times) would be stable for any length of time. And if not could it be stabilized…Just curious for myself. I am also given to understand that there are those reefers who dose Iron at measurable (including me) levels and it might be informative to us to know if Iron is impacted by sample storage problems that we saw with phosphorus.
As it turns out it appears Iron is impacted by sample storage (See Table 1 and Chart 1 & 2). Day 3 results, although within the test limits, show a downward tendency (20 ppb). It appears that nether the chlorine of the Nitric Acid had any stabilizing effect on the measurement. Day 7 shows a significant reduction in the measurement (70 ppb) for both the untreated sample and the chlorinated sample; however the Nitric Acid treated sample shows some indication of stabilization, but still a loss of some 30 ppb.
IODINE
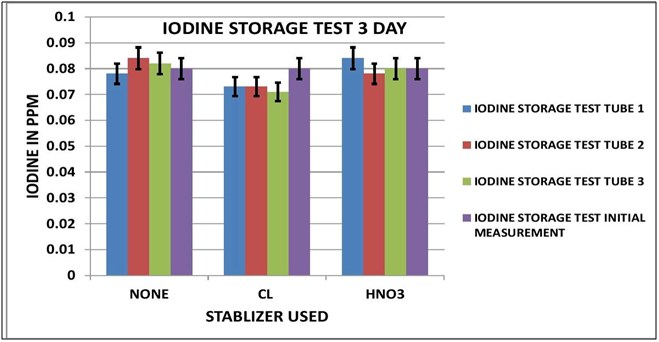
CHART 3
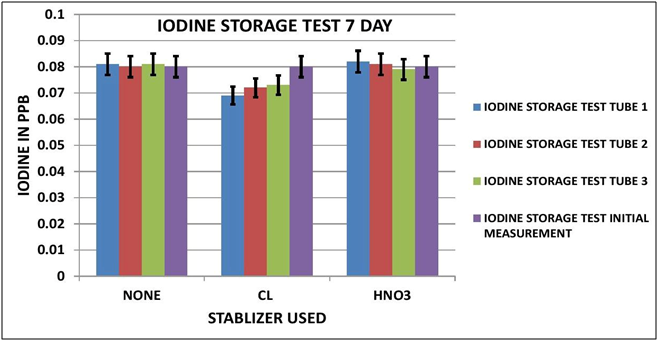
CHART 4
IODINE OBSERVATIONS
Looking at the Data Table 1 and Chart 3 & 4, it appears that Iodine is relatively stable during sample storage and there is no loss, at least for 7 days. The addition of Chlorine looks as if it might have a slight negative effect on the measurement; although it is not pronounced it is observable in both the 3 day and 7 day results. It appears the Nitric Acid has no effect.
PHOSPHOROUS
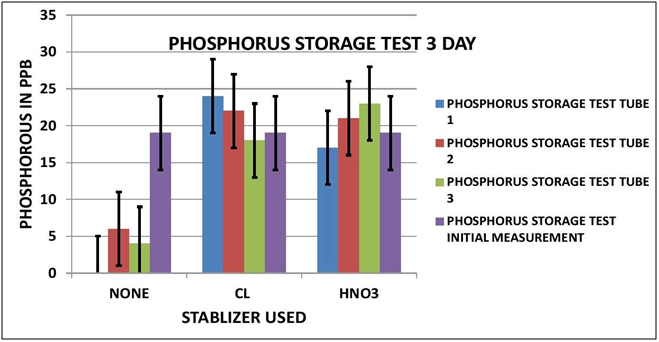
CHART 5
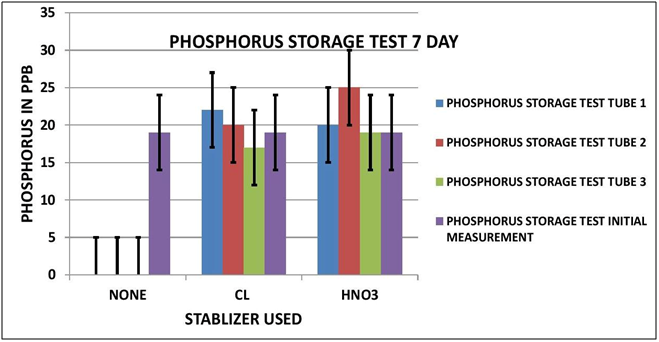
CHART 6
PHOSPHORUS OBSERVATIONS
Again looking at Data Table and Charts 5 & 6 we see the same type of results that we observed in the previous study. After 3 days with no stabilization the measured Phosphorous is significantly reduced to 3 ppm from 19 ppm. On day 7 there is none measurable. The addition of 7 ppm Chlorine stabilized the reading over the 7 day storage period. We can also see that the addition of 300 ppm Nitric Acid also provided measurement stability over the 7 day period.
REPLICATING ICP ANALYSIS EXPERIMENT
In my last post on this subject I ran an experiment to determine if stabilizing the sample would yield a closer agreement on the measurement of Phosphorus between the Hanna HI-736 and the ICP vendor’s results. The results of this initial experiment indicated that this would indeed be the case. There was much better agreement between the ICP results and my measurement with the HI-736. This second experiment was an attempt to replicate those results. This time I sent the samples to 3 ICP vendors (Vendors 1-3) and one Non-ICP test facility (Vendor 4). This might have been a mistake! In that it added some confusion to the work. On the other hand, it once again points out “Not all ICP Tests are created equal” .The results can be seen in TABLE 3 and CHART 7.
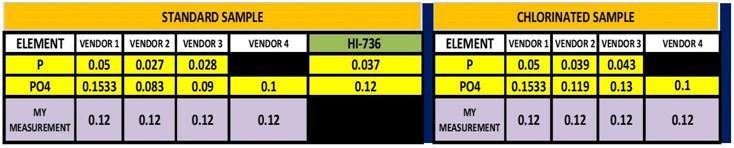
TABLE 3
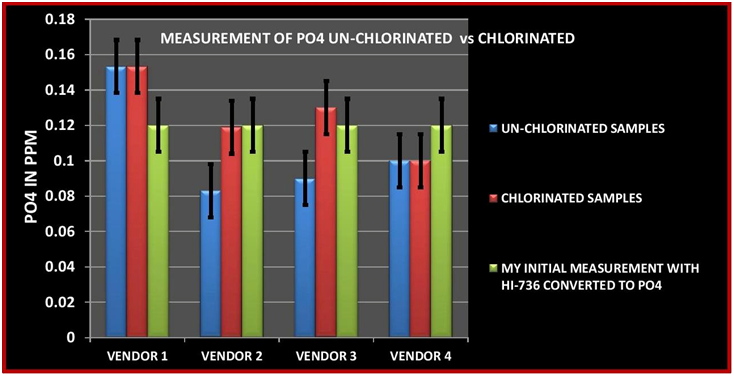
CHART 7
ICP REPLICATION EXPERIMENT OBSERVATIONS
The initial sample measurement is the average of 5 measurements made by me using the HI-736. In looking at the data table, 3 of the vendor’s measurement were lower and one was higher than my initial measurement in the unchlorinated sample. For the chlorinated sample two vendors reported no change and two reported almost identical values to my measurement. These results point out one of the challenges with ICP testing. That is same sample different vendor different results. This is not only true for the measurement of Phosphorus but other element. I have included the entire analysis results from all 4 vendors in TABLE 4. There have been a number of other posts and videos that have point out this same issue so I will not expand this just to say these outcomes present the dilemma of which one is correct? Vendor # 3 demonstrated the same tendency as vendor 2…Low measurement for the un-chlorinated sample and recovered measurement in the chlorinated sample. I am not entirely sure what to conclude from the results of Vendors 1 & 4. It is interesting to note that for both vendors the untreated and the chlorinated had exactly the same value. It is also interesting to note that vendor 4 was the closest value to the HI-736 results….I will leave it at that and ponder the results some more. All that being said, vendor # 2 was the same vendor that I used for the first set of experiments so I will conclude from the data that the ability to replicate the experiment was successful.
One other interesting note is the the magnitude the loss of PO4 from my first experiment to this experiment was roughly the same .04 ppm.
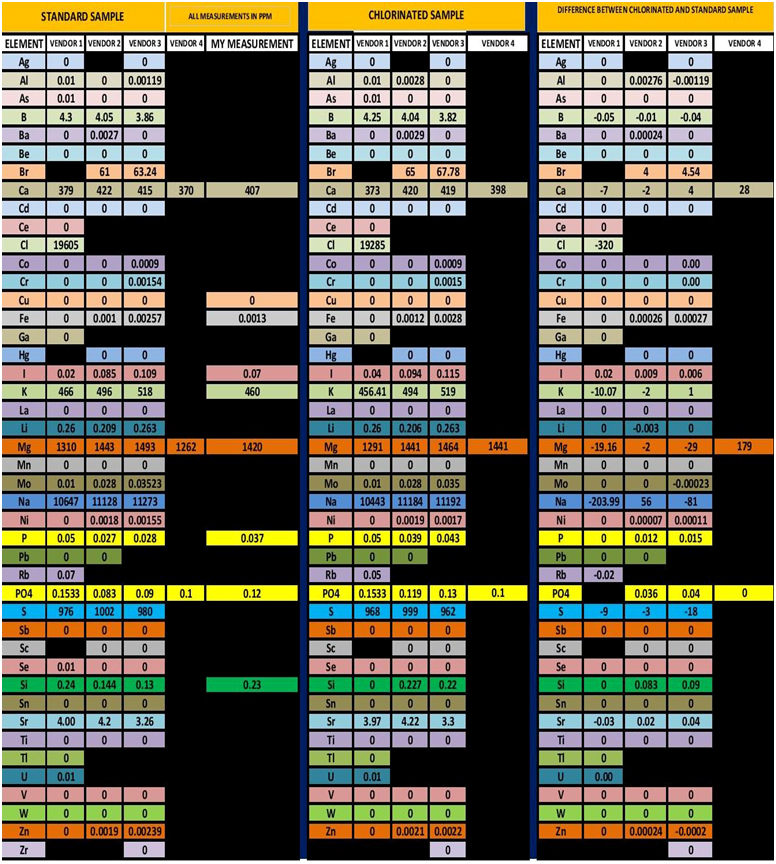
TABLE 4
All Measurement are in PPM
*NOTES ON NITRIC ACID EVALUATION
I AM NOT RECOMMENDING THE USE OF NITRIC ACID TO STABILIZE SAMPLES FOR TESTING, EITHER INTERNAL OR ESPECIALLY OUTSIDE TESTING. THE pH OF THE RESULTING SAMPLE AT 300PM IS QUITE LOW AN CAN PRESENT A POTENTIAL SAFETY HAZARD IF NOT HANDLED PROPERLY! THE EXPERIMENT WAS CONDUCTED TO EXPLORE A SPECIFIC QUESTION FROM THE PREVIOUS POST AND TO GAIN INSIGHT INTO THE ISSUE OF SAMPLE HANDLING AND STORAGE.
In some of the discussion in the previous post it was suggested that replacing Chlorine with Nitric Acid might also provide some stabilization to the samples. I set up several experiment to find out if: 1) Does the addition interfere with the measurement itself (the chemistry of the test) and 2) Does it provide a stabilizing effect and if so what is the minimum level required to do so. I will not go into any details about this work because that is not the purpose of this post, but if you would like to see the work just let me know and I will send it to you. The end results were that the Nitric Acid treatment at 300 ppm acted as a stabilizer for the stored samples. It actually appears to do a better job of stabilizing Iron than the chlorine in the 7 day storage test. During this evaluation on the use of Nitric Acid as a stabilizer, the initial measurement variability was very high. In discussions with @Dan_P and @taricha it was proposed that there could be fine suspended substrate kicked up by the nutrient cycle of my Vortechs that would be dissolved by the acidic environment and cause variability. Sure enough filtering the sample drastically reduced the variability. See the TABLE 5
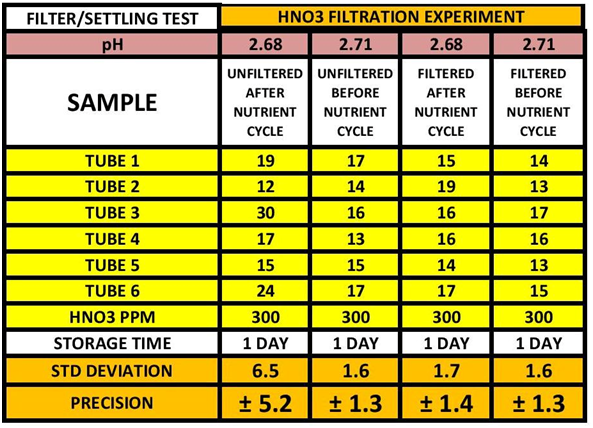
TABLE 5
CONCLUSIONS
So as I emerge from this “Rabbit Hole”, I find it has given me some insights into the world of measurement as it relates to testing of my reef tank. I would say the main insight for me is that: sample acquisition and handling is an important part of getting accurate results. This is true regardless of whether it is my own testing or outside testing services (ICP or other). Leaving samples setting for extended periods of time can in some cases yield inaccurate results. This has helped me to understand some confusing results that I was observing…. “Why when I went back and retested my retained samples were the measurements so different”…and “Why were my PO4 measurements consistently higher than the ICP test results of the same sample” These two questions were the drivers for my “trip down the rabbit hole”.
A second insight is the fact that the sample that I am taking is “biologically” active and some of the elements that I am measuring can be “tied up” or consumed during storage time. This is important to know because it will help me to better understand my measurement results. I have only looked at 3 of these elements, Phosphorus, Iodine and Iron. What other ones can be effected is yet unclear.
Well I think I will wrap this up. I believe that we have answered the 4 questions that we started out with:
Congratulations if you have made it this far!!! I know this is a lot to take in, but I hope it is useful to some…Thanks for sticking with it.

Rick
In my last post in July of last year I presented the case that samples sent for ICP analysis were not completely stable and that during the storage time before testing there was a change (reduction) in the measurable level of Phosphorous and thus the reported PO4 values from the ICP test results were consistently lower than my measurements using the Hanna Tester. This was demonstrated by several experiments including sampling to an ICP vendor and replicating the results. Through additional experiments it was found that a 7ppm add of bleach to the samples would stabilize the samples and keep the measurable amount phosphorous from being reduced. This was also demonstrated via several experiments as well as ICP test results. If you are interested in the details here is a link to the post.

SAMPLE STORAGE AND ITS IMPACT ON PO4 MEASUREMENT AND ICP MEASUREMENT RESULTS (PART 2)
My First Trip To Wonderland!...Down the Rabbit Hole! My first trip to Wonderland started in late 2019 when I started experimenting to find the answer to a question that had been troubling me for over 2 years. Since the very beginning of this project it has been my goal to understand why my...
 www.reef2reef.com
www.reef2reef.com
At the end of the post I mentioned that based on the results of this work there were additional questions to be explored as well as some proposed questions from the comments to the post. This trip to “Wonderland” attempts to address some of these.
Questions and Issues Explored
- Is Iodine measurement impacted by storage
- Is Iron measurement impacted by storage
- Will an acidic environment stabilize the sample
- Can the ICP experiment be replicated
Thanks to @Dan_P and @taricha for their help. As always their contributions were of great value!
IODINE---IRON---PHOSPHORUS STORAGE IMPACT EXPERIMENT
EXPERIMENTAL LAYOUT
A large sample of tank water was collected. The sample was then filtered through a 20 micron filter to remove any suspended material. (See Note on Nitric Acid Evaluation)* Part of the sample was separated out and “spiked” with Iron to raise the level to a measurable level. Measurements were made on this initial sample for Iron, Iodine and Phosphorus. There were 5 measurements made on each and an average taken to represent the “INITIAL MEASUREMENT” value.
The two samples (Iron Spiked & Un-Spiked) were each separated into 3 sample sets. One sample set would be the baseline (NO PRESERVATIVE): The second set was treated with 7ppm Cl: The third set was treated with 300 ppm Nitric Acid (See Note on Nitric Acid Evaluation)*. That would be 6 sample sets altogether. The six sets were then placed in 15 mL sample tubes and place in “storage” at room temperature 70⁰ F (21 C) The sample flow chart below will better explain this…I think
At the end of 3 days of storage samples were removed and measured for: Iron, Iodine, and Phosphorus. Three measurements were taken for each of the test sample sets. That would be 9 measurements for each of the Elements…3 for No Preservative…3 for the Chlorine…and 3 for the Nitric Acid…27 sample measurements total for the 3 day storage samples. The very same protocol was done for day 7.
TEST METHODS
Iron was tested using the Hanna HI-746 Low Range Iron Checker. It has a reported accuracy of ±20 ppb ± 5% of the reading.
Iodine was tested by a method I developed using the Red Sea Chemistry and HI-707 Low Range Nitrite Checker. It has an error of ± 5% of the reading (Here is a link to the method. https://www.reef2reef.com/threads/using-hanna-checker-hi-707-to-test-for-iodine.669022/ )
Phosphorus was tested using the Hanna HI-736 ULR Phosphorus Checker. It has a reported accuracy of ±5ppb ± 5% of the reading.
The error ranges are reflected in the error bars included on the charts.
The results can be seen in TABLE 1 CHARTS 1-6.
TABLE 1
IRON
CHART 1
CHART 2
IRON OBSERVATIONS
The levels of Iron in natural seawater are very low, .006 ppb. This level is well below where we as hobbyist would be able to measure with any level of confidence. The HI-764 has a stated accuracy of ± 20 ppb which is many times the natural level. Also Iron is very transient in the aquarium. It is used up very quickly so even if you are dosing iron, it will be quickly depleted in the tank. So you might be asking yourself “Why did you include Iron in this experiment?” I would say mostly curiosity! I wanted to see if elevated levels of iron (> 90 times) would be stable for any length of time. And if not could it be stabilized…Just curious for myself. I am also given to understand that there are those reefers who dose Iron at measurable (including me) levels and it might be informative to us to know if Iron is impacted by sample storage problems that we saw with phosphorus.
As it turns out it appears Iron is impacted by sample storage (See Table 1 and Chart 1 & 2). Day 3 results, although within the test limits, show a downward tendency (20 ppb). It appears that nether the chlorine of the Nitric Acid had any stabilizing effect on the measurement. Day 7 shows a significant reduction in the measurement (70 ppb) for both the untreated sample and the chlorinated sample; however the Nitric Acid treated sample shows some indication of stabilization, but still a loss of some 30 ppb.
IODINE
CHART 3
CHART 4
IODINE OBSERVATIONS
Looking at the Data Table 1 and Chart 3 & 4, it appears that Iodine is relatively stable during sample storage and there is no loss, at least for 7 days. The addition of Chlorine looks as if it might have a slight negative effect on the measurement; although it is not pronounced it is observable in both the 3 day and 7 day results. It appears the Nitric Acid has no effect.
PHOSPHOROUS
CHART 5
CHART 6
PHOSPHORUS OBSERVATIONS
Again looking at Data Table and Charts 5 & 6 we see the same type of results that we observed in the previous study. After 3 days with no stabilization the measured Phosphorous is significantly reduced to 3 ppm from 19 ppm. On day 7 there is none measurable. The addition of 7 ppm Chlorine stabilized the reading over the 7 day storage period. We can also see that the addition of 300 ppm Nitric Acid also provided measurement stability over the 7 day period.
REPLICATING ICP ANALYSIS EXPERIMENT
In my last post on this subject I ran an experiment to determine if stabilizing the sample would yield a closer agreement on the measurement of Phosphorus between the Hanna HI-736 and the ICP vendor’s results. The results of this initial experiment indicated that this would indeed be the case. There was much better agreement between the ICP results and my measurement with the HI-736. This second experiment was an attempt to replicate those results. This time I sent the samples to 3 ICP vendors (Vendors 1-3) and one Non-ICP test facility (Vendor 4). This might have been a mistake! In that it added some confusion to the work. On the other hand, it once again points out “Not all ICP Tests are created equal” .The results can be seen in TABLE 3 and CHART 7.
TABLE 3
CHART 7
ICP REPLICATION EXPERIMENT OBSERVATIONS
The initial sample measurement is the average of 5 measurements made by me using the HI-736. In looking at the data table, 3 of the vendor’s measurement were lower and one was higher than my initial measurement in the unchlorinated sample. For the chlorinated sample two vendors reported no change and two reported almost identical values to my measurement. These results point out one of the challenges with ICP testing. That is same sample different vendor different results. This is not only true for the measurement of Phosphorus but other element. I have included the entire analysis results from all 4 vendors in TABLE 4. There have been a number of other posts and videos that have point out this same issue so I will not expand this just to say these outcomes present the dilemma of which one is correct? Vendor # 3 demonstrated the same tendency as vendor 2…Low measurement for the un-chlorinated sample and recovered measurement in the chlorinated sample. I am not entirely sure what to conclude from the results of Vendors 1 & 4. It is interesting to note that for both vendors the untreated and the chlorinated had exactly the same value. It is also interesting to note that vendor 4 was the closest value to the HI-736 results….I will leave it at that and ponder the results some more. All that being said, vendor # 2 was the same vendor that I used for the first set of experiments so I will conclude from the data that the ability to replicate the experiment was successful.
One other interesting note is the the magnitude the loss of PO4 from my first experiment to this experiment was roughly the same .04 ppm.
TABLE 4
All Measurement are in PPM
*NOTES ON NITRIC ACID EVALUATION
I AM NOT RECOMMENDING THE USE OF NITRIC ACID TO STABILIZE SAMPLES FOR TESTING, EITHER INTERNAL OR ESPECIALLY OUTSIDE TESTING. THE pH OF THE RESULTING SAMPLE AT 300PM IS QUITE LOW AN CAN PRESENT A POTENTIAL SAFETY HAZARD IF NOT HANDLED PROPERLY! THE EXPERIMENT WAS CONDUCTED TO EXPLORE A SPECIFIC QUESTION FROM THE PREVIOUS POST AND TO GAIN INSIGHT INTO THE ISSUE OF SAMPLE HANDLING AND STORAGE.
In some of the discussion in the previous post it was suggested that replacing Chlorine with Nitric Acid might also provide some stabilization to the samples. I set up several experiment to find out if: 1) Does the addition interfere with the measurement itself (the chemistry of the test) and 2) Does it provide a stabilizing effect and if so what is the minimum level required to do so. I will not go into any details about this work because that is not the purpose of this post, but if you would like to see the work just let me know and I will send it to you. The end results were that the Nitric Acid treatment at 300 ppm acted as a stabilizer for the stored samples. It actually appears to do a better job of stabilizing Iron than the chlorine in the 7 day storage test. During this evaluation on the use of Nitric Acid as a stabilizer, the initial measurement variability was very high. In discussions with @Dan_P and @taricha it was proposed that there could be fine suspended substrate kicked up by the nutrient cycle of my Vortechs that would be dissolved by the acidic environment and cause variability. Sure enough filtering the sample drastically reduced the variability. See the TABLE 5
TABLE 5
CONCLUSIONS
So as I emerge from this “Rabbit Hole”, I find it has given me some insights into the world of measurement as it relates to testing of my reef tank. I would say the main insight for me is that: sample acquisition and handling is an important part of getting accurate results. This is true regardless of whether it is my own testing or outside testing services (ICP or other). Leaving samples setting for extended periods of time can in some cases yield inaccurate results. This has helped me to understand some confusing results that I was observing…. “Why when I went back and retested my retained samples were the measurements so different”…and “Why were my PO4 measurements consistently higher than the ICP test results of the same sample” These two questions were the drivers for my “trip down the rabbit hole”.
A second insight is the fact that the sample that I am taking is “biologically” active and some of the elements that I am measuring can be “tied up” or consumed during storage time. This is important to know because it will help me to better understand my measurement results. I have only looked at 3 of these elements, Phosphorus, Iodine and Iron. What other ones can be effected is yet unclear.
Well I think I will wrap this up. I believe that we have answered the 4 questions that we started out with:
- Is Iodine measurement impacted by storage
- Is Iron measurement impacted by storage
- Will an acidic environment stabilize the sample
- Can the ICP experiment be replicated
Congratulations if you have made it this far!!! I know this is a lot to take in, but I hope it is useful to some…Thanks for sticking with it.
Rick
Last edited:


















