My First Trip To Wonderland!...Down the Rabbit Hole!

My first trip to Wonderland started in late 2019 when I started experimenting to find the answer to a question that had been troubling me for over 2 years. Since the very beginning of this project it has been my goal to understand why my measurement results, in particular PO4, differed from ICP results. I could not explain this difference with what I knew about colorimetric and ICP measurements. I have stated in previous posts that I had been using ICP as the “Target” for my own testing. The goal was to improve my measurement methods using my test kits and measurement protocols. For almost 2 years I have been following this path, but the issue with my measurement and ICP results persisted.
In recent post by @4sylvester he cited a very nice article that nicely covers the subject “Phosphorus Determination in Waters and Extracts of Soils and By-Products: Inductively Coupled Plasma Spectrometry versus Colorimetric Procedures” If you are interested here is a link post (Post # 100 here https://www.reef2reef.com/threads/icp-test-results-vs-hobby-grade.733197/page-5
This quote from that article describes my understanding of the relationship between ICP measurements and colorimetric measurement such as the Hanna Checkers.
"The method of P determination used in water samples and extracts of soils and by-products can influence the reported concentrations. For most sample types, ICP is slightly greater than colorimetric analyses, but the two methods are well correlated for high P samples. However, extreme caution needs to be used for samples with low P concentrations (e.g., 60 mg/kg extractable P), as the difference may be as great as 5 fold. "
This is exactly what I expected when I sent in my first ICP test and compared it to my Hanna results, but to my surprise the ICP reported a lower value for P and PO4....not by a small amount but by close to 50% (My measurement 20 ppb P ....ICP 10 ppb P) I concluded that my measurement was incorrect so I went back to my retained sample of the test I sent for ICP analysis and retested....Sure enough I got a lower reading 12 ppb----close enough! So that should have been the end of the story, but it was not. I continued to send in ICP tests every 90 days (Started in 2017) and I saw exactly the same results...Not every time but by far the majority.... ICP lower P than Hanna Hi-736 ...Retest the retain...results are close to ICP results.
Quoting from Alice In Wonderland

Call me a slow learner ....It took me almost 2 years to stop and ask the question what is going on? I am either the world’s worst (but consistent) Tester or there is something I am missing. I had seen on this forum several discussions about the same observation...ICP tests lower then Hanna Tester and didn't really follow them until @taricha made a statement that caught my attention "maybe the sample is not stable" ...That set me down this "Rabbit Hole"....And literally 100's of PO4 tests later here we are...My first posting ( https://www.reef2reef.com/threads/sample-storage-and-its-impact-on-phosphate-measurement.696800/) was the beginning of my trip to "Wonderland" but only the beginning. I basically set out to understand what impact storing a sample has on my test results... My basic conclusion for myself was not to store samples for any length of time in any type of container....Which is as it turns out was well known before I went down the "Rabbit Hole"....My bad, should have done more looking...none the less I got really good at the test, ....But it still did not answer my question "Why do my ICP tests come out consistently lower than my Hanna results?" According to my understanding this should not be happening and if I read the above reference paper correctly they agree...Should not be lower only higher or at best equal to.
Does ICP vendor testing overcome these sampling issues? Can a vendor hold a sample for several days and still get the same test results? I can for sure say my testing methods cannot. I contend that this sampling problem does impact ICP test results for some elements. Somehow, way beyond my understanding, it has to do with Bio-activity and Biofilm that make some amount of P unavailable to the current ICP test protocols...This is the focus of my second trip to "Wonderland"….Which you are now reading…Welcome to my trip to Wonderland


Once again I would like to thank @taricha and @Dan_P for their help on this project. Their questions, challenges and expertise along with their supportive experimental results were very helpful in keeping the project on track and out of too many “rabbit holes”
OUTLINE:
CONFIRMATION EXPERIMENT
In order to be confident that what I had done in previous work was reproducible I did a replica of the initial experiment on PO4. A large sample was taken from my tank and divided into fifteen 15mL Polypropylene (PP) sample tubes. Initial measurements were taken on Day 0 (3 tubes). Then every 3 days measurements were taken on the next 3 tubes. This was repeated up to day 12. The results can be seen in Chart 1.
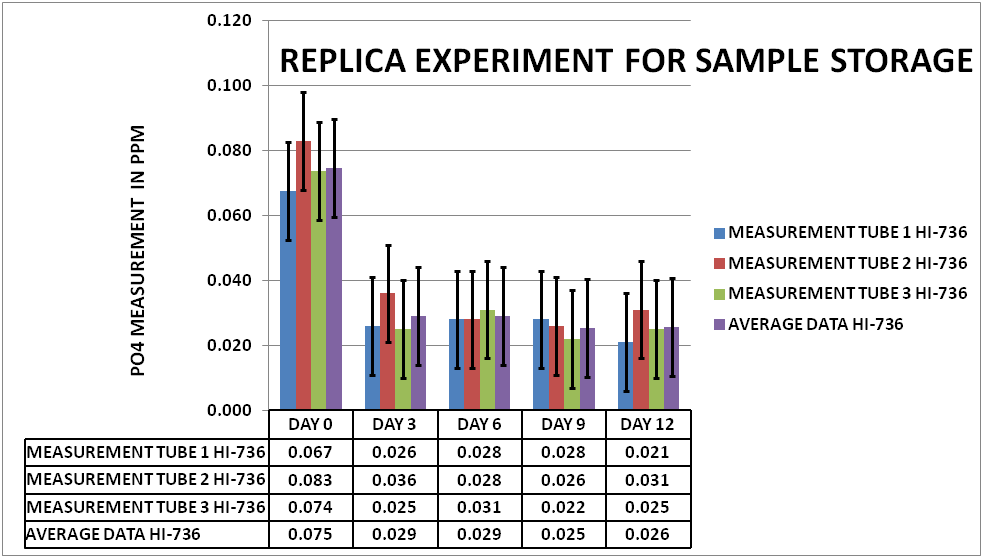
CHART 1
As you can see the depletion on the measured PO4 was on average .049 ppm or about a 35% reduction in the measurable PO4 . This level of depletion is greater than the Hanna 95% confidence interval (represented by the black vertical bars). This would indicate that the initial experiments could be replicated and the depletion effect is a real signal and not noise.
So just like the Cheshire Cat in Wonderland….Is he there or not!

TEMPERATURE EFFECT
One of the questions that arose in the last set of experiments was whether this is a biological effect related to the bio-active materials contained in the tank water. It was suggested by @taricha that if it was Bio-Active that cooling the sample down during storage should show a slowing down of the depletion. An experiment was set up in which a large sample from my tank was separated into 2 sample sets. Each set consisted of eighteen 15mL PP Sample Tubes. One sample set was placed in a refrigerator at 42⁰ F (5.5⁰ C). The second set of samples was stored at 78⁰F (25.5⁰ C). Initial measurements were taken on day 0 using a HI-736 as well as an HI-774. The samples were stored for 6 days. Measurements were taken on day 3 and 6. The results can be seen in Chart 2
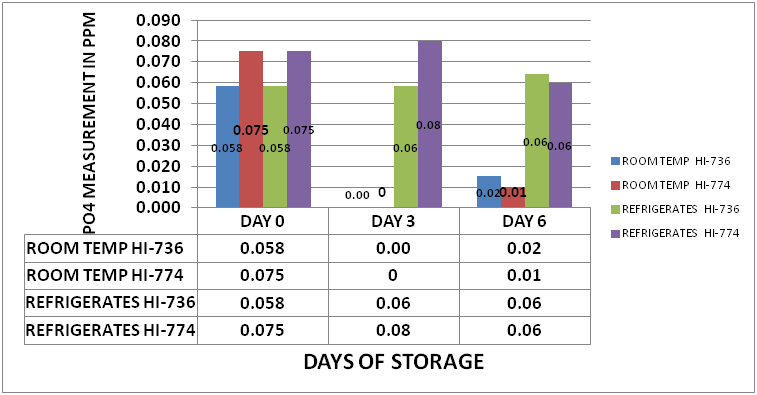
CHART 2
As the charts and the data clearly shows, cooling the sample down during storage virtually stops the depletion of measured PO4. This provides a fairly strong hint that the depletion process could be biological in nature.
CHLORINATION EFFECT
Along with the temperature effect an experiment was set up to look at the effect of sanitizing the sample with hypochlorite. First two separate experiments were conducted to determine if hypochlorite would interfere with the Hanna PO4-P (HI-736) test measurement. @taricha concluded up to 35 ppm showed no interference. I went up to 20 ppm and found the same result…He was braver than I was! The bleach solution used in the experiment 5% lab grade Sodium Hypochlorite that at the 15 ppm level tested “0” P on the Hanna HI-736.
Having concluded that the chlorine would not interfere with the measurement an experiment was set up to evaluate the effect of chlorination the sample before storage. I selected a chlorine level of 15 ppm as my starting point. A large sample was taken from my tank and divided into 2 samples. The first sample was the control…no hypochlorite added. The second sample was spiked with 15 ppm hypochlorite. The two sample sets were then placed in 15 mL PP sample tubes. 15 unchlorinated and 15 chlorinated. Initial measurements were taken on day 0 then measurements taken every 3 days for a total of 12 days storage. The results can be seen in Chart 3
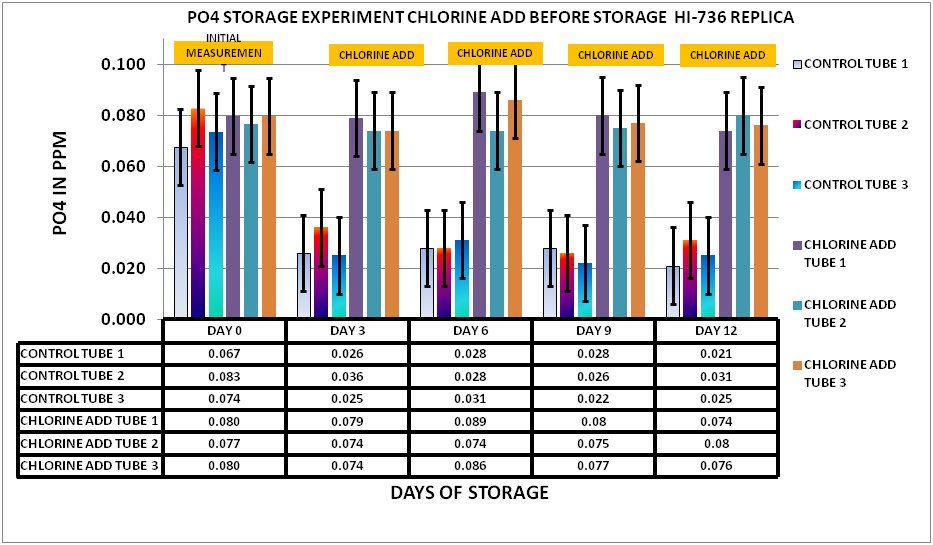
CHART 3
As you can see from the charts and data the chlorine treated sample remain stable over the storage period maintaining measured values roughly equivalent to the initial reading on Day 0, whereas the untreated sample showed a significant reduction over the same storage period. Once again the evidence points to biological activity (Biofilm) contributing to the PO4 depletion
Going deeper into the rabbit hole I set out to test whether the ICP measurement of P also showed a difference between chlorinated and unchlorinated samples. Many believe that no matter what happens to the sample during shipment, the ICP method will detect the true concentration of elements. Is this true even if an element such as phosphorous in the form of PO4 is assimilated by a bio film on the sample container surface? Would the ICP result for P be higher when bio film formation is inhibited? And would the magnitude of difference in ICP results between chlorinated and unchlorinated samples be comparable to the PO4 depletion measured with the Hanna Checker?
An experiment essentially the same as my previous chlorination experiment described above was conducted. In this experiment 3 samples were sent to an ICP vendor for measurement.
The description of the samples and the results of the can be seen in CHART 4
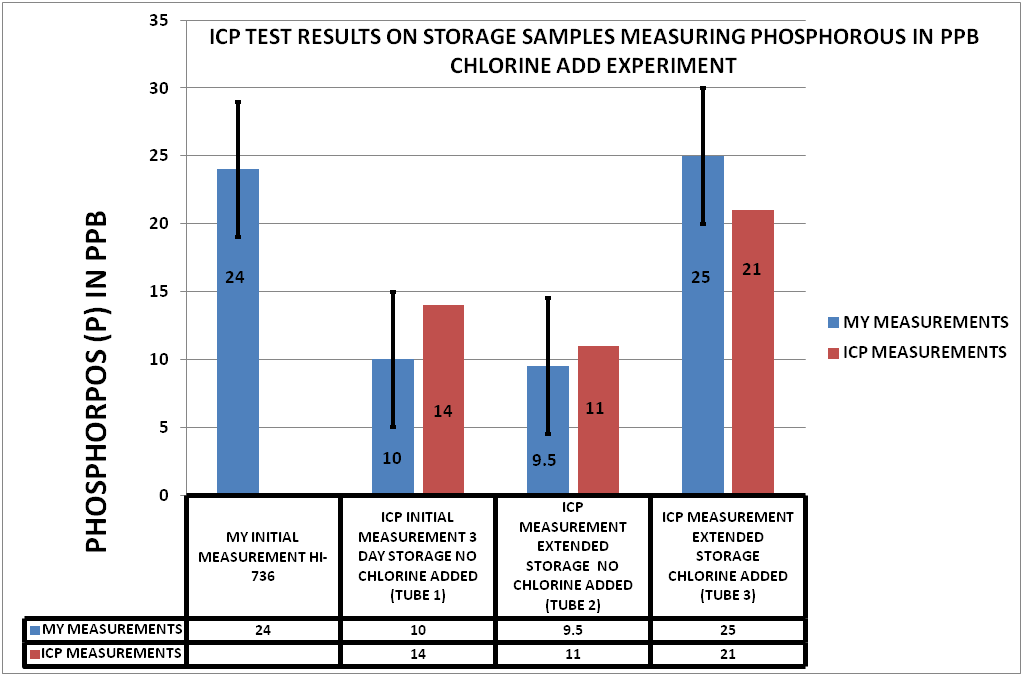
CHART 4
CHART 4 SAMPLE IDENTIFICATION
Tube 1 was an unchlorinated sample that was shipped to the vendor the same day the sample was taken. The sample was 3 days in transit before testing (3 Days of Storage) (INITIAL ICP MEASUREMENT)
Tube 2 was an unchlorinated sample that was held for several days before being shipped to the vendor. (EXTENDED STORAGE NO CHLORINE)
Tube 3 was a chlorinated sample (15 ppm) that was also held the same number of days as tube 2 before shipment to the vendor. (EXTENDED STORAGE CHLORINATED)
I retained samples of each of these to be measured on the HI-736. These are the values on Chart 4 labeled MY MEASUREMENTS
All of the ICP samples as well as the retains came from the same tank sample just as in the previous experiment.
Is it true that even if an element such as phosphorous in the form of PO4 is assimilated by a bio film on the sample container surface, ICP would still detect it?
Would the ICP result for P be higher when bio film formation is inhibited?
And would the magnitude of difference in ICP results between chlorinated and unchlorinated samples be comparable to the PO4 depletion measured with the Hanna Checker?
A statistical sidebar:
When comparing two experimental treatments, it requires knowing how much variability there is in the results. Without this knowledge, we don’t know whether an observed difference is a result of the treatment or chance. In the comparisons above, we know about the variation in the Hanna measurement of PO4 and we are forced to assume a variation in the ICP measurements. In this case, we will assume the ICP variation is small based on the similarity ICP results of Tubes 1 and 2.
SUMMARY
In summary, this small experiment provides us with evidence that the ICP measurement of PO4 is not what we expected. The ICP method does not detect all the P in the sample. When PO4 is lost to what the data suggests is biological activity, for example, a bio film on the container surface, the ICP method fails to detect it. What we don’t know is whether other forms of P are also lost to the bio film.
This experimental data also suggests there can be good agreement between the Hanna Checker and ICP measurements when the samples contain the same amount of PO4 (Tube 1, 2 and 3 results), which occurred in this experiment when the measurements were made after the depletion of PO4 (Tubes 1 or2 ) and when no depletion occurred (Tube 3). Usually, we expect the ICP total P measurement to be higher than observed P-PO4 measurement of the Hanna because we assume that other forms of P exist in our systems. In this case, the P in my water appears to be dominated by PO4. Is this true in other systems as well? We will never know unless ICP measurements are improved.
SOME OTHER INTERESTING OUTCOMES
@Dan_P conducted several experiment at lower levels of PO4 (.06-.17 ppm PO4) as well as elevated levels (.3+ ppm PO4). The results can be seen in CHART 5.
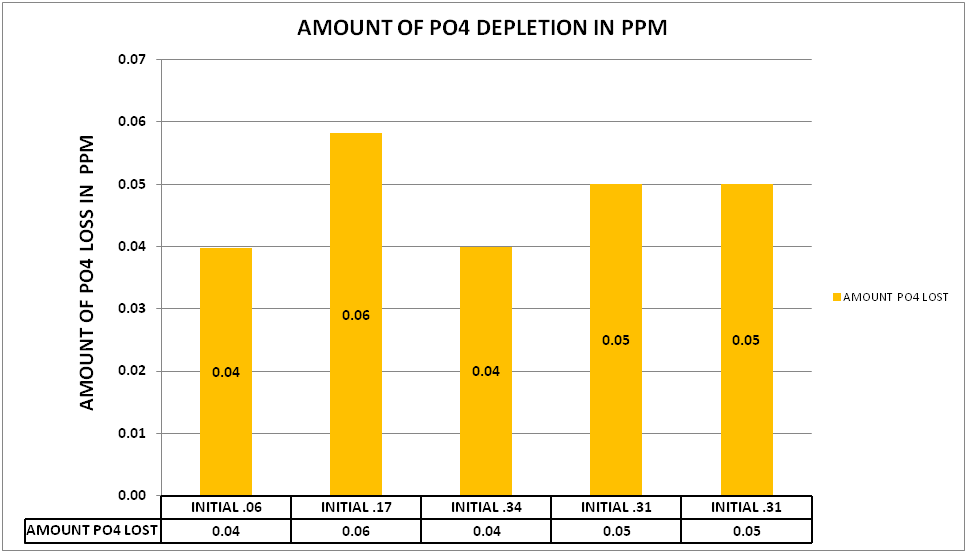
CHART 5
What is interesting about these results is there appears to be a limit to the amount of the PO4 depletion. The higher initial levels of .17 to .34 had the same level of PO4 depletion as the lower level of .06. This model also fits the data generated in my experiments as well as experiments conducted by @taricha.
@Dan_P then raised the question “does this relate to the SA/V ratio effect observed in earlier experiments?” The idea being that the 15 mL tubes have a given surface area and the Biofilm may be limited by this surface area. He conducted an experiment to test this idea. , He cut one of the storage test tubes in quarters down its length. He then put two quarters in each of two test tubes. So, he added to each sample, half of a test tube. Since both inside and outside was in contact with the sample, the surface was the same as adding one entire test tube, thus doubling the surface area.
After 5 days he measured the results. The results can be seen in CHART 6
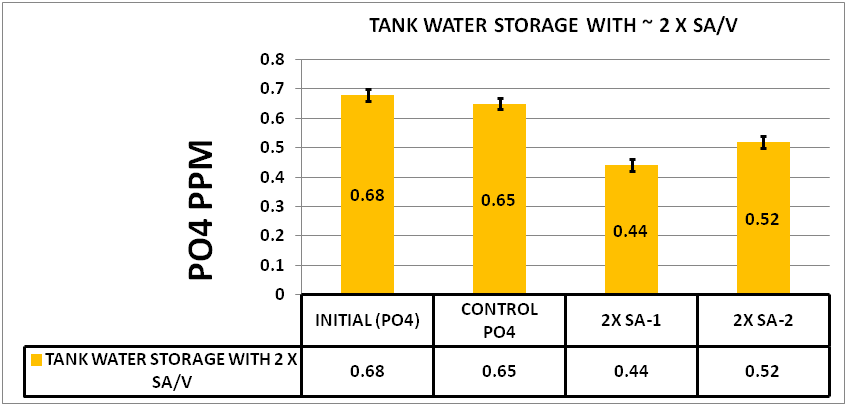
CHART 6
GENERAL OBSERVATIONS
I do not characterize the experimental results as an indication of ICP measurement issue, but an overall protocol issue relating to sample handling. This holds true regardless of the type of testing one is conducting. We talk about measurements in terms of method: ICP, Hanna Checkers and Hobby Grade Kits because that is what we use. Every test requires a good sampling protocol in order to get a measurement that is representative of the total volume or batch. As the article from the link above clearly highlights, water sampling is no trivial issue....Can't just "dip and go”. One has to consider type of container, preservation requirements etc. based on the elements you are testing. As is pointed out some elements and compounds are "rugged" and can be handled accordingly but others like P...PO4.. NO2 are much more demanding in the sample handling...This is like a whole science unto itself!
There is a great deal more to be looked at (more rabbit holes). The chart below poses some potential additional analytes to look into.
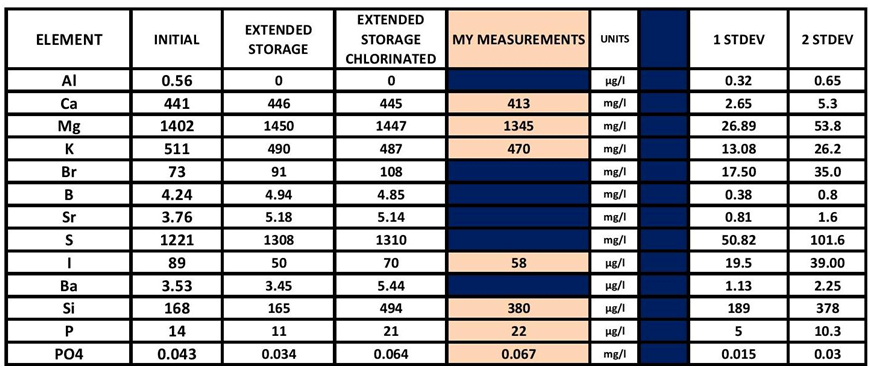
THIS CHART IS A PARTIAL LIST OR THE RESULTS FROM THE ICP ANALYSIS DONE ON THE CHLORINE ADD EXPERIMENT
Thank you for following me into Wonderland…. could be we will meet here again!
rick
PS: During this project I found an interesting gadget that does the sample shaking for you. It frees up your hands to do other things while the sample is shaking…
Here is a video of it in action: You need to mix for 15-20 seconds before you place it on the shaker to keep the reagent from clumping …but then shake away!!
(link to Video)
I found it on Amazon but it is listed several other places including ebay..
My first trip to Wonderland started in late 2019 when I started experimenting to find the answer to a question that had been troubling me for over 2 years. Since the very beginning of this project it has been my goal to understand why my measurement results, in particular PO4, differed from ICP results. I could not explain this difference with what I knew about colorimetric and ICP measurements. I have stated in previous posts that I had been using ICP as the “Target” for my own testing. The goal was to improve my measurement methods using my test kits and measurement protocols. For almost 2 years I have been following this path, but the issue with my measurement and ICP results persisted.
In recent post by @4sylvester he cited a very nice article that nicely covers the subject “Phosphorus Determination in Waters and Extracts of Soils and By-Products: Inductively Coupled Plasma Spectrometry versus Colorimetric Procedures” If you are interested here is a link post (Post # 100 here https://www.reef2reef.com/threads/icp-test-results-vs-hobby-grade.733197/page-5
This quote from that article describes my understanding of the relationship between ICP measurements and colorimetric measurement such as the Hanna Checkers.
"The method of P determination used in water samples and extracts of soils and by-products can influence the reported concentrations. For most sample types, ICP is slightly greater than colorimetric analyses, but the two methods are well correlated for high P samples. However, extreme caution needs to be used for samples with low P concentrations (e.g., 60 mg/kg extractable P), as the difference may be as great as 5 fold. "
This is exactly what I expected when I sent in my first ICP test and compared it to my Hanna results, but to my surprise the ICP reported a lower value for P and PO4....not by a small amount but by close to 50% (My measurement 20 ppb P ....ICP 10 ppb P) I concluded that my measurement was incorrect so I went back to my retained sample of the test I sent for ICP analysis and retested....Sure enough I got a lower reading 12 ppb----close enough! So that should have been the end of the story, but it was not. I continued to send in ICP tests every 90 days (Started in 2017) and I saw exactly the same results...Not every time but by far the majority.... ICP lower P than Hanna Hi-736 ...Retest the retain...results are close to ICP results.
Quoting from Alice In Wonderland
Call me a slow learner ....It took me almost 2 years to stop and ask the question what is going on? I am either the world’s worst (but consistent) Tester or there is something I am missing. I had seen on this forum several discussions about the same observation...ICP tests lower then Hanna Tester and didn't really follow them until @taricha made a statement that caught my attention "maybe the sample is not stable" ...That set me down this "Rabbit Hole"....And literally 100's of PO4 tests later here we are...My first posting ( https://www.reef2reef.com/threads/sample-storage-and-its-impact-on-phosphate-measurement.696800/) was the beginning of my trip to "Wonderland" but only the beginning. I basically set out to understand what impact storing a sample has on my test results... My basic conclusion for myself was not to store samples for any length of time in any type of container....Which is as it turns out was well known before I went down the "Rabbit Hole"....My bad, should have done more looking...none the less I got really good at the test, ....But it still did not answer my question "Why do my ICP tests come out consistently lower than my Hanna results?" According to my understanding this should not be happening and if I read the above reference paper correctly they agree...Should not be lower only higher or at best equal to.
Does ICP vendor testing overcome these sampling issues? Can a vendor hold a sample for several days and still get the same test results? I can for sure say my testing methods cannot. I contend that this sampling problem does impact ICP test results for some elements. Somehow, way beyond my understanding, it has to do with Bio-activity and Biofilm that make some amount of P unavailable to the current ICP test protocols...This is the focus of my second trip to "Wonderland"….Which you are now reading…Welcome to my trip to Wonderland
Once again I would like to thank @taricha and @Dan_P for their help on this project. Their questions, challenges and expertise along with their supportive experimental results were very helpful in keeping the project on track and out of too many “rabbit holes”
OUTLINE:
- CONFIRMATION EXPERIMENTS
- TEMPERATURE EFFECT
- CHLORINATION EFFECT
- MY RESULTS
- ICP TEST RESULTS
- SUMMARY
- SOME OTHER INTERESTING OUTCOMES
- DISCUSSION
CONFIRMATION EXPERIMENT
In order to be confident that what I had done in previous work was reproducible I did a replica of the initial experiment on PO4. A large sample was taken from my tank and divided into fifteen 15mL Polypropylene (PP) sample tubes. Initial measurements were taken on Day 0 (3 tubes). Then every 3 days measurements were taken on the next 3 tubes. This was repeated up to day 12. The results can be seen in Chart 1.
CHART 1
As you can see the depletion on the measured PO4 was on average .049 ppm or about a 35% reduction in the measurable PO4 . This level of depletion is greater than the Hanna 95% confidence interval (represented by the black vertical bars). This would indicate that the initial experiments could be replicated and the depletion effect is a real signal and not noise.
So just like the Cheshire Cat in Wonderland….Is he there or not!
TEMPERATURE EFFECT
One of the questions that arose in the last set of experiments was whether this is a biological effect related to the bio-active materials contained in the tank water. It was suggested by @taricha that if it was Bio-Active that cooling the sample down during storage should show a slowing down of the depletion. An experiment was set up in which a large sample from my tank was separated into 2 sample sets. Each set consisted of eighteen 15mL PP Sample Tubes. One sample set was placed in a refrigerator at 42⁰ F (5.5⁰ C). The second set of samples was stored at 78⁰F (25.5⁰ C). Initial measurements were taken on day 0 using a HI-736 as well as an HI-774. The samples were stored for 6 days. Measurements were taken on day 3 and 6. The results can be seen in Chart 2
CHART 2
As the charts and the data clearly shows, cooling the sample down during storage virtually stops the depletion of measured PO4. This provides a fairly strong hint that the depletion process could be biological in nature.
CHLORINATION EFFECT
My Results
Along with the temperature effect an experiment was set up to look at the effect of sanitizing the sample with hypochlorite. First two separate experiments were conducted to determine if hypochlorite would interfere with the Hanna PO4-P (HI-736) test measurement. @taricha concluded up to 35 ppm showed no interference. I went up to 20 ppm and found the same result…He was braver than I was! The bleach solution used in the experiment 5% lab grade Sodium Hypochlorite that at the 15 ppm level tested “0” P on the Hanna HI-736.
Having concluded that the chlorine would not interfere with the measurement an experiment was set up to evaluate the effect of chlorination the sample before storage. I selected a chlorine level of 15 ppm as my starting point. A large sample was taken from my tank and divided into 2 samples. The first sample was the control…no hypochlorite added. The second sample was spiked with 15 ppm hypochlorite. The two sample sets were then placed in 15 mL PP sample tubes. 15 unchlorinated and 15 chlorinated. Initial measurements were taken on day 0 then measurements taken every 3 days for a total of 12 days storage. The results can be seen in Chart 3
CHART 3
As you can see from the charts and data the chlorine treated sample remain stable over the storage period maintaining measured values roughly equivalent to the initial reading on Day 0, whereas the untreated sample showed a significant reduction over the same storage period. Once again the evidence points to biological activity (Biofilm) contributing to the PO4 depletion
ICP Results
Going deeper into the rabbit hole I set out to test whether the ICP measurement of P also showed a difference between chlorinated and unchlorinated samples. Many believe that no matter what happens to the sample during shipment, the ICP method will detect the true concentration of elements. Is this true even if an element such as phosphorous in the form of PO4 is assimilated by a bio film on the sample container surface? Would the ICP result for P be higher when bio film formation is inhibited? And would the magnitude of difference in ICP results between chlorinated and unchlorinated samples be comparable to the PO4 depletion measured with the Hanna Checker?
An experiment essentially the same as my previous chlorination experiment described above was conducted. In this experiment 3 samples were sent to an ICP vendor for measurement.
The description of the samples and the results of the can be seen in CHART 4
CHART 4
CHART 4 SAMPLE IDENTIFICATION
Tube 1 was an unchlorinated sample that was shipped to the vendor the same day the sample was taken. The sample was 3 days in transit before testing (3 Days of Storage) (INITIAL ICP MEASUREMENT)
Tube 2 was an unchlorinated sample that was held for several days before being shipped to the vendor. (EXTENDED STORAGE NO CHLORINE)
Tube 3 was a chlorinated sample (15 ppm) that was also held the same number of days as tube 2 before shipment to the vendor. (EXTENDED STORAGE CHLORINATED)
I retained samples of each of these to be measured on the HI-736. These are the values on Chart 4 labeled MY MEASUREMENTS
All of the ICP samples as well as the retains came from the same tank sample just as in the previous experiment.
The answers to our questions from the result of this experiment and observations:
Is it true that even if an element such as phosphorous in the form of PO4 is assimilated by a bio film on the sample container surface, ICP would still detect it?
No. Phosphorous concentration as determined by ICP is lower in the two samples not treated with sodium hypochlorite than the one that is. Moreover, this difference in ICP measurement is comparable to that measured by the Hanna Checker. These results suggest that for my samples, phosphorous is mostly in the form of PO4.
Would the ICP result for P be higher when bio film formation is inhibited?
Yes, at least within the limits of the small sample size of this experiment. P can be “lost“ to the ICP method during transit.
And would the magnitude of difference in ICP results between chlorinated and unchlorinated samples be comparable to the PO4 depletion measured with the Hanna Checker?
Yes, but again within the limits of the small sample size of this experiment. This might be the case only when most of the phosphorous in the sample is in the form of PO4. Further testing would be needed to determine whether organophosphorous compounds are also subject to assimilation by biofilms.
A statistical sidebar:
When comparing two experimental treatments, it requires knowing how much variability there is in the results. Without this knowledge, we don’t know whether an observed difference is a result of the treatment or chance. In the comparisons above, we know about the variation in the Hanna measurement of PO4 and we are forced to assume a variation in the ICP measurements. In this case, we will assume the ICP variation is small based on the similarity ICP results of Tubes 1 and 2.
SUMMARY
In summary, this small experiment provides us with evidence that the ICP measurement of PO4 is not what we expected. The ICP method does not detect all the P in the sample. When PO4 is lost to what the data suggests is biological activity, for example, a bio film on the container surface, the ICP method fails to detect it. What we don’t know is whether other forms of P are also lost to the bio film.
This experimental data also suggests there can be good agreement between the Hanna Checker and ICP measurements when the samples contain the same amount of PO4 (Tube 1, 2 and 3 results), which occurred in this experiment when the measurements were made after the depletion of PO4 (Tubes 1 or2 ) and when no depletion occurred (Tube 3). Usually, we expect the ICP total P measurement to be higher than observed P-PO4 measurement of the Hanna because we assume that other forms of P exist in our systems. In this case, the P in my water appears to be dominated by PO4. Is this true in other systems as well? We will never know unless ICP measurements are improved.
SOME OTHER INTERESTING OUTCOMES
INITIAL LEVEL OF PO4 AND ITS EFFECT ON DEPLETION
@Dan_P conducted several experiment at lower levels of PO4 (.06-.17 ppm PO4) as well as elevated levels (.3+ ppm PO4). The results can be seen in CHART 5.
CHART 5
What is interesting about these results is there appears to be a limit to the amount of the PO4 depletion. The higher initial levels of .17 to .34 had the same level of PO4 depletion as the lower level of .06. This model also fits the data generated in my experiments as well as experiments conducted by @taricha.
@Dan_P then raised the question “does this relate to the SA/V ratio effect observed in earlier experiments?” The idea being that the 15 mL tubes have a given surface area and the Biofilm may be limited by this surface area. He conducted an experiment to test this idea. , He cut one of the storage test tubes in quarters down its length. He then put two quarters in each of two test tubes. So, he added to each sample, half of a test tube. Since both inside and outside was in contact with the sample, the surface was the same as adding one entire test tube, thus doubling the surface area.
After 5 days he measured the results. The results can be seen in CHART 6
CONFIRMATION OF SURFACE AREA TO VOLUME EFFECT
CHART 6
As you can see the increase in the SA/V increased the depletion significantly over that of the .03 ppm seen in the control. This appears to support Dan’s idea that one of the factors limiting the amount of the depletion of the PO4 is the Surface Area to Volume ratio.GENERAL OBSERVATIONS
- Saltwater tank water samples are not stable over time with respect to PO4 measurements made using Colorimetric testing methods (Hanna Checkers). It appears from the data that within 3 days (72 hours) the level of depletion is large enough to cause issues with the measurement results…IMO…. 10-20 ppb P/ .03-.06 PPM PO4 . This fact is well known in the science of water testing and there are very specific procedure required for storage and preservation of samples. These requirements are element and compound specific. …If you are interested in the details of this here is a link https://apps.who.int/iris/handle/10665/41851
- ICP testing appears to be impacted by the sample storage in the same way. This is evidenced by the fact that with the addition of 15 ppm chlorine added an additional 7-10 ppb phosphorous when measured vs the same sample without chlorine.
- The depletion amount appears to be limited by the SA/V of the sample container (Tube). I looks as if it is somewhere around .06 ppm PO4/ 20 ppb P. More experimentation will be needed to better define this value.
- The addition of 15 ppm chlorine appears to stabilize the PO4 measurement for an extended storage time when being measured by colorimetric methods (Hanna Checker).
- The addition of 15 ppm chlorine appears to impact the results of an ICP test measurement of total P in the sample.
I do not characterize the experimental results as an indication of ICP measurement issue, but an overall protocol issue relating to sample handling. This holds true regardless of the type of testing one is conducting. We talk about measurements in terms of method: ICP, Hanna Checkers and Hobby Grade Kits because that is what we use. Every test requires a good sampling protocol in order to get a measurement that is representative of the total volume or batch. As the article from the link above clearly highlights, water sampling is no trivial issue....Can't just "dip and go”. One has to consider type of container, preservation requirements etc. based on the elements you are testing. As is pointed out some elements and compounds are "rugged" and can be handled accordingly but others like P...PO4.. NO2 are much more demanding in the sample handling...This is like a whole science unto itself!
There is a great deal more to be looked at (more rabbit holes). The chart below poses some potential additional analytes to look into.
THIS CHART IS A PARTIAL LIST OR THE RESULTS FROM THE ICP ANALYSIS DONE ON THE CHLORINE ADD EXPERIMENT
Thank you for following me into Wonderland…. could be we will meet here again!
rick
PS: During this project I found an interesting gadget that does the sample shaking for you. It frees up your hands to do other things while the sample is shaking…
Here is a video of it in action: You need to mix for 15-20 seconds before you place it on the shaker to keep the reagent from clumping …but then shake away!!
(link to Video)
I found it on Amazon but it is listed several other places including ebay..
Last edited:



















