Show some pics of the setup, interested to know how it works out!
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Say you could slowly dose dry calcium hydroxide (kalk) in a reef tank water mixing chamber.....
- Thread starter Lousybreed
- Start date
- Tagged users None
Then it seems you don't understand how calcium hydroxide works.I appreciate it, but I am not interested. I want to get rid of all carbonic acid. It would be too expensive at my system size (700 gallons). I am setting up my experiment right now!
First Calcium hydroxide is two ions the first obviously is Calcium the second not so obviously is hydroxide or more specifically base. This is essentially bleach plus calcium. When you put this in RO then the base part raises the PH to a high level as though you added bleach and the extra ion is Calcium. Our tanks don't just need Calcium they also need carbonate or bicarbonate. Carbonic acid is H+(aq) + CO3(aq). If you could strip the acid from the carbonic acid then you can get the carbonate or alkalinity. Enter Calcium Hydroxide. Viola Calcium and Carbonate in solution without precipitation.
The problem with your idea is that you will not have enough carbonic acid from the air to compensate for the all of the hydroxide (base) you are dumping in your tank, no matter how slowly. When the tank reaches critical mass, I believe to be around PH 8.5, then the calcium will start reacting with the the existing free carbonate in your tank and precipitate out in insoluble calcium carbonate. This will cause what the old timers call calcium storm. Nearly all of your carbonate will bind and precipitate out. Leaving you with hardly any alk or calcium left. Your PH will remain high. Needless to say this will be the end of your tank. Just dosing it slowly won't stop this process from coming because you will not be able to get enough CO2 in your system to neutralize the hydroxide.
If you don't care about this tank then by all means, this is a great experiment on how this works. If you do care about the tank I would highly advise against it.
P.S. I've done this.
Thank you for your input. I do understand calcium hydroxide. With my pH of my tank I can tell you I do have enough carbonic acid for it to react with. But considering the mass precipitation event I will take it slow!!!!Then it seems you don't understand how calcium hydroxide works.
First Calcium hydroxide is two ions the first obviously is Calcium the second not so obviously is hydroxide or more specifically base. This is essentially bleach plus calcium. When you put this in RO then the base part raises the PH to a high level as though you added bleach and the extra ion is Calcium. Our tanks don't just need Calcium they also need carbonate or bicarbonate. Carbonic acid is H+(aq) + CO3(aq). If you could strip the acid from the carbonic acid then you can get the carbonate or alkalinity. Enter Calcium Hydroxide. Viola Calcium and Carbonate in solution without precipitation.
The problem with your idea is that you will not have enough carbonic acid from the air to compensate for the all of the hydroxide (base) you are dumping in your tank, no matter how slowly. When the tank reaches critical mass, I believe to be around PH 8.5, then the calcium will start reacting with the the existing free carbonate in your tank and precipitate out in insoluble calcium carbonate. This will cause what the old timers call calcium storm. Nearly all of your carbonate will bind and precipitate out. Leaving you with hardly any alk or calcium left. Your PH will remain high. Needless to say this will be the end of your tank. Just dosing it slowly won't stop this process from coming because you will not be able to get enough CO2 in your system to neutralize the hydroxide.
If you don't care about this tank then by all means, this is a great experiment on how this works. If you do care about the tank I would highly advise against it.
P.S. I've done this.
Dude I am already dosing kalk. No problem. So your saying that everyone that doses kalk will have a precipitation event unless your pH is 6.5? All I am talking about is dosing your alk needs in kalk over 24 hrs in a slightly more concentrated slurry. Drop by drop. I am not talking about dumping in a half cup of calcium hydroxide every morning!
- Joined
- Sep 21, 2018
- Messages
- 6,675
- Reaction score
- 7,169
It will work if the pH around the dissolving particle of calcium hydroxide stays below say pH 10. So what you need is a very high mixing rate. A single particle of calcium hydroxide drifting in seawater might still cause a precipitate in the boundary layer around the particle, but a particle racing through seawater might have a very thin boundary layer which might stay below pH10.Ok please hear me out. Let’s just say you found this amazingly precise dry powder metering gizmo where you could fill a small hopper with dry kalk (aka calcium hydroxide) and it would meter the powder into a mixing chamber that contained your reef tank water. @Randy Holmes-Farley I know you mentioned if you dumped it in too fast it would cause precipitation of mag and whatnot due to the massive spike in pH. If you assume you have a slow addition all day long and your flow rate in your mixer was 500gph.....is this something that could possibly work?
You are looking for high turbulence not high flow.
A power drill with a paint mixer attachment stirring a small volume of seawater as solid calcium is slowly added is the direction to head. An inline static mixer with high flow would be another approach.
Why don’t you run a pilot to determine whether you have sufficient turbulence in your mixing chamber.
And because you don't understand the chemistry and because of that you are going to get a calcium storm.Dude I am already dosing kalk. No problem. So your saying that everyone that doses kalk will have a precipitation event unless your pH is 6.5? All I am talking about is dosing your alk needs in kalk over 24 hrs in a slightly more concentrated slurry. Drop by drop. I am not talking about dumping in a half cup of calcium hydroxide every morning!
When Kalkwasser came into the hobby about 30 years ago there were many stories of people doing this. What you are doing has been tried many many times. If you want to try it again, then it is your tank. I am just trying to explain why what you are doing won't work. I have given you the chemistry. I have told you that I have had personal experience doing this.
What you decide to do with this information is up to you. There is a reason that a lot of the ways that we deal with calcium hydroxide today are the way the are. I guess it is incumbent on every new generation of reefers to find out for themselves.
As I said. Good luck.
I think this is a far better idea than putting your entire tank on the line.Why don’t you run a pilot to determine whether you have sufficient turbulence in your mixing chamber.
Adding Calcium Hydroxide to RODI water is not the same as adding Calcium Hydroxide to tank water.Dude I am already dosing kalk. No problem. So your saying that everyone that doses kalk will have a precipitation event unless your pH is 6.5? All I am talking about is dosing your alk needs in kalk over 24 hrs in a slightly more concentrated slurry. Drop by drop. I am not talking about dumping in a half cup of calcium hydroxide every morning!
This is exactly what I am talking about. I even found the article where RFH talks about how you can do it this way. You are right. I will be adding the drip by drip slurry into an area with high mixing turbulence in my sump. I am going to start small and go from there. Like make the representative slurry and drip it with a dropper to see how it goes.It will work if the pH around the dissolving particle of calcium hydroxide stays below say pH 10. So what you need is a very high mixing rate. A single particle of calcium hydroxide drifting in seawater might still cause a precipitate in the boundary layer around the particle, but a particle racing through seawater might have a very thin boundary layer which might stay below pH10.
You are looking for high turbulence not high flow.
A power drill with a paint mixer attachment stirring a small volume of seawater as solid calcium is slowly added is the direction to head. An inline static mixer with high flow would be another approach.
Why don’t you run a pilot to determine whether you have sufficient turbulence in your mixing chamber.
Attachments
I am a chemical engineer and trust me I get it. I guess that I often tone down my questions here looking to see what people think about my idea. . I appreciate your thoughts. Here is my question to you. Are you suggesting that NO MATTER how you add it you will see precipitation if you use a slurry? Like 5 ml per min in a sump that has 4000gph turn over that will have two 2,500gph wavemakers pointed opposing to each other where the slurry will be dripped? I have a hard time with absolutes, especially when it comes to chemistry. The literature I have read suggests that if you keep the localized pH below 10 you should not have precipitation. Randy has a publication even stating this is an acceptable method of using alk as long as you don’t raise the pH too fast.And because you don't understand the chemistry and because of that you are going to get a calcium storm.
When Kalkwasser came into the hobby about 30 years ago there were many stories of people doing this. What you are doing has been tried many many times. If you want to try it again, then it is your tank. I am just trying to explain why what you are doing won't work. I have given you the chemistry. I have told you that I have had personal experience doing this.
What you decide to do with this information is up to you. There is a reason that a lot of the ways that we deal with calcium hydroxide today are the way the are. I guess it is incumbent on every new generation of reefers to find out for themselves.
As I said. Good luck.
The reason I challenge these assumptions is that I find a lack of data behind these assumptions. The more I dive in the easier hit seems to be. For example I make my own trace element mixes. I was told I will get cancer, there is no way you will figure this out, you will kill your corals, etc. I am currently making trace elements solutions for my 700 gallon system for a whopping 25 cents per month. And my ICP tests have never been better (still room for improvement however).
I am a chemical engineer and trust me I get it. I guess that I often tone down my questions here looking to see what people think about my idea. . I appreciate your thoughts. Here is my question to you. Are you suggesting that NO MATTER how you add it you will see precipitation if you use a slurry? Like 5 ml per min in a sump that has 4000gph turn over that will have two 2,500gph wavemakers pointed opposing to each other where the slurry will be dripped? I have a hard time with absolutes, especially when it comes to chemistry. The literature I have read suggests that if you keep the localized pH below 10 you should not have precipitation. Randy has a publication even stating this is an acceptable method of using alk as long as you don’t raise the pH too fast.
The reason I challenge these assumptions is that I find a lack of data behind these assumptions. The more I dive in the easier hit seems to be. For example I make my own trace element mixes. I was told I will get cancer, there is no way you will figure this out, you will kill your corals, etc. I am currently making trace elements solutions for my 700 gallon system for a whopping 25 cents per month. And my ICP tests have never been better (still room for improvement however).
Something to note with BRS pharma Calcium Hydroxide is that with daily mixing in a reactor, it never fully clarifies. Their Kalk is a very fine powder and probably stays suspended. So my dosing with that certainly delivers a little undissolved Kalk (or some percipitates) with a slow drip. I suspect it may be adding more than the anticipated .25dkh and causing my Alk to slightly rise over time.
I did a little experiment just now. I took a gallon of tank water, hooked a wavemaker up to it, and slow dripped 1 ml of 3% kalk slurry into it.....no precipitation as of yet. Still monitoring it. I am going to do a bigger 10 gallon experiment tomorrow. And also dose my 20l that is sitting around.
I also did he exact same experiment with no wavemaker in it. I can tell you that one did have precipitationI did a little experiment just now. I took a gallon of tank water, hooked a wavemaker up to it, and slow dripped 1 ml of 3% kalk slurry into it.....no precipitation as of yet. Still monitoring it. I am going to do a bigger 10 gallon experiment tomorrow. And also dose my 20l that is sitting around.
Ok now I have a better understanding.I am a chemical engineer and trust me I get it. I guess that I often tone down my questions here looking to see what people think about my idea. . I appreciate your thoughts. Here is my question to you. Are you suggesting that NO MATTER how you add it you will see precipitation if you use a slurry? Like 5 ml per min in a sump that has 4000gph turn over that will have two 2,500gph wavemakers pointed opposing to each other where the slurry will be dripped? I have a hard time with absolutes, especially when it comes to chemistry. The literature I have read suggests that if you keep the localized pH below 10 you should not have precipitation. Randy has a publication even stating this is an acceptable method of using alk as long as you don’t raise the pH too fast.
The reason I challenge these assumptions is that I find a lack of data behind these assumptions. The more I dive in the easier hit seems to be. For example I make my own trace element mixes. I was told I will get cancer, there is no way you will figure this out, you will kill your corals, etc. I am currently making trace elements solutions for my 700 gallon system for a whopping 25 cents per month. And my ICP tests have never been better (still room for improvement however).
I used to be software engineer and now I trade stocks. I have 35 years of experience with salt water aquariums and I use chemistry to get in trouble and do crazy, stupid, things.
My knowledge is empirical. Here is what I have seen. As @Dan_P mentioned there is a localized problem with putting in concentrated CaOH. IME this is less of a problem. If there is a precipitation event is usually short lived and easily recovered. This is only true in the beginning of your dosing process. As you dose this way more and more the tank's ph begins to rise, I think. Back when I did this I didn't test for ph, so I can't say for sure. I also wasn't watching alk closely, possibly the alk will start dropping. Anyway the tank becomes more unstable. Then one day the local precipitation that was no big deal spirals into a full on cloudy tank storm. Suffice it to say this is a catastrophic event.
I was speaking earlier about the amount of carbonic acid in your system. Your coral demand is going to determine your dosing needs. If you keep up with the coral demand and ignore the carbonic acid and it becomes unavailable I think that is where the tank begins to become unstable.
Because your tank is so large you have the advantage of this process possibly taking much longer to the point that it might not happen. I don't know I haven't every had a tank this big.
The tough thing about dosing directly and not with RO is that the CaOH is very concentrated. You could probably calculate the equivalent CaOH to either your consumption or your current Kalkwasser supplementation. That will at least give you a place to start. I believe the reason that people started using the top off method was to limit the amount of CaOH entering the system. This is a natural limiter and I think it keeps amounts below dangerous levels, so that people could feel comfortable that they haven't gone too far.
Also Randy pointed out in my thread that MgOH is more soluble than CaCO2 and that higher ph levels will start to precipitate out Mg. Consumer grade Mg tests are crap so it is hard to use them to get real Mg readings to know if you are doing this. I would only trust ICP for Mg and as you know those aren't the fastest feedback in the world. You can dose for the Mg, but be aware this could happen.
I like to do crazy things because how else will things move forward.
I would like to know the gizmo that you are using to dose with. I would like to automate the process that I am using and my only solution so far is making my own powder doser. Something I don't relish designing or working the kinks out of. So if it is kewl do share if you can, it would be much appreciated.
This is how we learn!!!! Ok I am going to probably start dosing a suspension of kalk. The dry dosing is WAAAAAY too expensive. I got my first quote back (verbal actually) and it would be in the ball park of $8,000 and still it was too big. So now my next step is to suspend the kalk in RODI.....and meter that in with a high precision dosing pump. At 5ml per min that is like 10-20 drops per minute. Picture massive flow in my sump where the drips are going. To keep the kalk in suspension I will be using a Sicce 3.0 pump and a closed loop circ system to ensure that the kalk cannot settle on the bottom. This will be housed in a 70 liter pharma grade storage bin (gasket sealed, virgin plastic, etc). I will be installing a small CO2 scrubber to remove CO2 as the slurry is used (I want to limit the surface calcium carbonate formation) as the level is drawn down. Now for the cool part. I link this to my Apex and when the pH hits 8.3 it cuts lower to the doser. Everything I have read is that once you let the pH get to the 8.4 and above mark you can get into real trouble. So I will automate to have my calcium reactor run during the peak pH times and my kalk slurry gizmo to run during the lower pH times. Basically 16 hrs on kalk and 8 hrs on calcium reactor. The slurry I am liking the most is a very dilute 3% kalk, 97% RODI.Ok now I have a better understanding.
I used to be software engineer and now I trade stocks. I have 35 years of experience with salt water aquariums and I use chemistry to get in trouble and do crazy, stupid, things.
My knowledge is empirical. Here is what I have seen. As @Dan_P mentioned there is a localized problem with putting in concentrated CaOH. IME this is less of a problem. If there is a precipitation event is usually short lived and easily recovered. This is only true in the beginning of your dosing process. As you dose this way more and more the tank's ph begins to rise, I think. Back when I did this I didn't test for ph, so I can't say for sure. I also wasn't watching alk closely, possibly the alk will start dropping. Anyway the tank becomes more unstable. Then one day the local precipitation that was no big deal spirals into a full on cloudy tank storm. Suffice it to say this is a catastrophic event.
I was speaking earlier about the amount of carbonic acid in your system. Your coral demand is going to determine your dosing needs. If you keep up with the coral demand and ignore the carbonic acid and it becomes unavailable I think that is where the tank begins to become unstable.
Because your tank is so large you have the advantage of this process possibly taking much longer to the point that it might not happen. I don't know I haven't every had a tank this big.
The tough thing about dosing directly and not with RO is that the CaOH is very concentrated. You could probably calculate the equivalent CaOH to either your consumption or your current Kalkwasser supplementation. That will at least give you a place to start. I believe the reason that people started using the top off method was to limit the amount of CaOH entering the system. This is a natural limiter and I think it keeps amounts below dangerous levels, so that people could feel comfortable that they haven't gone too far.
Also Randy pointed out in my thread that MgOH is more soluble than CaCO2 and that higher ph levels will start to precipitate out Mg. Consumer grade Mg tests are crap so it is hard to use them to get real Mg readings to know if you are doing this. I would only trust ICP for Mg and as you know those aren't the fastest feedback in the world. You can dose for the Mg, but be aware this could happen.
I like to do crazy things because how else will things move forward.Just be aware of that if you lose control of this process or you make a mistake it could be catastrophic. Plan accordingly.
I would like to know the gizmo that you are using to dose with. I would like to automate the process that I am using and my only solution so far is making my own powder doser. Something I don't relish designing or working the kinks out of. So if it is kewl do share if you can, it would be much appreciated.
Not easy to explain. But I will make a drawing to make sense of it. Basically my 70 liter reservoir will be filled with RODI and kalk at the 97% and 3% ratio of water and kalk. Since this is a fill and use (batch process really) I can maintain precise control over my slurry density compared to an auto top-off system. This slurry is then dripped into the sump to mix and then back to the tank.
I did a little experiment and dribbled 3ml of the 3% slurry into my tank. I cannot believe it but the pH did go up a bit!!!!
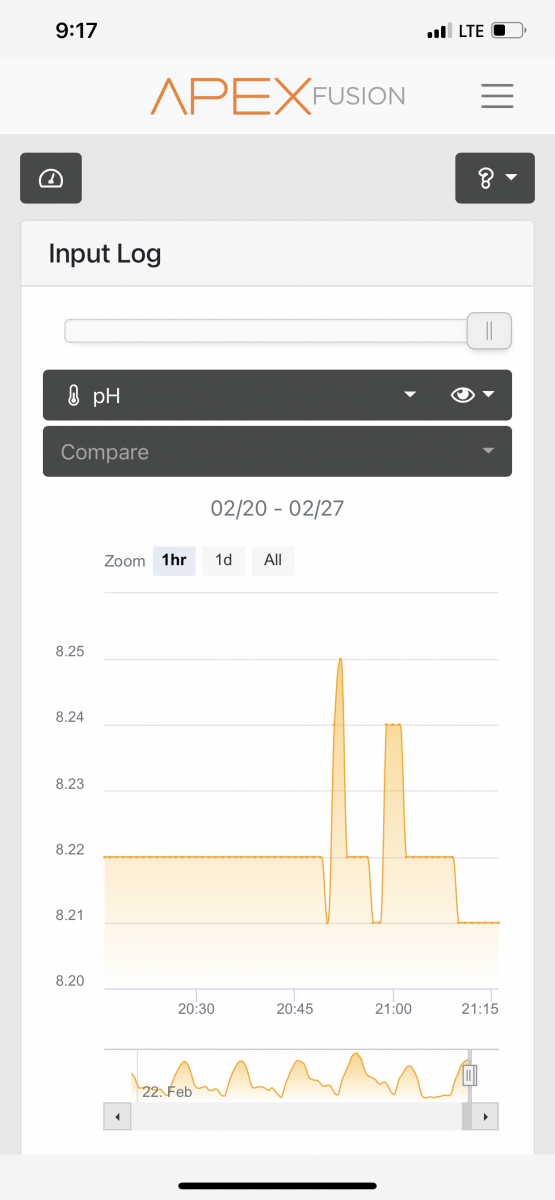
One last note, the part about tanks doing fine for a while and then going into overdose mode is very interesting and something we need to watch closely. This is good info and I can see how that could happen. Still hoping the man himself will touch in on this topic! Dr RHF!
Ok, software is where I can speak. Be VERY VERY careful with feedback loops. Maybe a second PH probe is in order. If one piece of what you doing screws up ... and it will. You want to have some sort of precautions built in to deal with it. Redundancy is key here.
PH is a good pinch point to ensure levels don't get out of hand. I think that what you are going to find is that you will not be using as much of slurry as you think you will. It doesn't take very much to make things happen.
Also just as an aside. I am not sure what got on you on this contraption, but there are cleaner ways to deal with this. Please don't think that this is me saying don't do what you are doing. I ABSOLUTELY think you should, because the learning is worth way more than just buying something and using it without really understanding it. The way that I deal with Kalk is with a Kalk stirrer and a doser. I use my other method as filler for what the Kalk can't keep up with. Just a thought.
I think you are on to it. Keep posting it will be a fun ride for sure.
PH is a good pinch point to ensure levels don't get out of hand. I think that what you are going to find is that you will not be using as much of slurry as you think you will. It doesn't take very much to make things happen.
Also just as an aside. I am not sure what got on you on this contraption, but there are cleaner ways to deal with this. Please don't think that this is me saying don't do what you are doing. I ABSOLUTELY think you should, because the learning is worth way more than just buying something and using it without really understanding it. The way that I deal with Kalk is with a Kalk stirrer and a doser. I use my other method as filler for what the Kalk can't keep up with. Just a thought.
I think you are on to it. Keep posting it will be a fun ride for sure.
Similar threads
-
- AMS: Article
- Replies
- 61
- Views
- 4,098
- Replies
- 6
- Views
- 340
- Poll
- Replies
- 25
- Views
- 1,651
-
- AMS: Article
- Replies
- 34
- Views
- 2,549



















