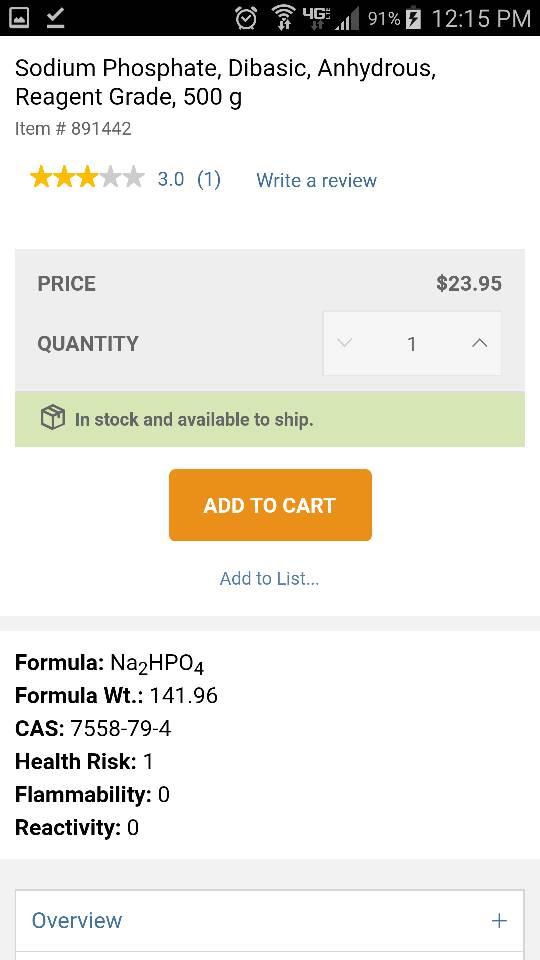
Hey Randy,
Is this product okay to mix in RO and dose into my reef tank to raise phosphate levels? There were several formulations available but this had the shortest molecular formula so I thought that equalled purest (not sure if that checks out haha).
Also, how would I determine how much to dose to raise my tank by a certain ppm (or ppb, I have the Hanna ULR phosphorus which reads ppb and I have to convert to ppm phosphate).
Thanks!

Is this product okay to mix in RO and dose into my reef tank to raise phosphate levels? There were several formulations available but this had the shortest molecular formula so I thought that equalled purest (not sure if that checks out haha).
Also, how would I determine how much to dose to raise my tank by a certain ppm (or ppb, I have the Hanna ULR phosphorus which reads ppb and I have to convert to ppm phosphate).
Thanks!