It's cheaperIt’s fine to do that, or just baking soda, but it’s no clear advantage over using AFR to do it.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Starting Tropic Marin All-For-Reef without adjust calcium and alkalinity first?
- Thread starter Soilworker
- Start date
- Tagged users None
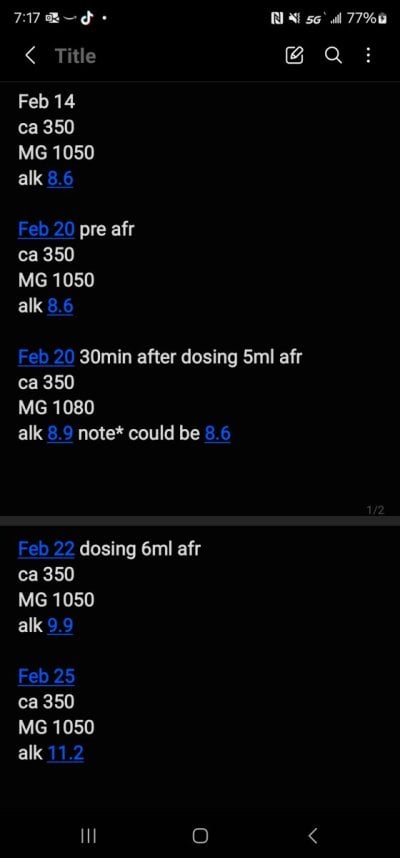
afr has done nothing for my tank but raise my alk super high. I'm using salifert as my test. what should I do? my tank took a big hit the other day. all my corals and my clam closed up. looked ok the next day but they are not 100%. did a 10% w/c. won't be dosing anything for a while.
Attachments
You must be overdosing.afr has done nothing for my tank but raise my alk super high. I'm using salifert as my test. what should I do? my tank took a big hit the other day. all my corals and my clam closed up. looked ok the next day but they are not 100%. did a 10% w/c. won't be dosing anything for a while.
Stop dosing completely until the Alk declines to where you want it.
When it is at that point, start dosing following the dosing instuctions on the bottle or using the online calculator for your size tank.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,238
- Reaction score
- 63,591
Agreed . If alk is rising you are dosing too much.
I've only been dosing the recommended amount. I've noticed that the only thing that rises is the alk. the ca and MG stayed about the same.You must be overdosing.
Stop dosing completely until the Alk declines to where you want it.
When it is at that point, start dosing following the dosing instuctions on the bottle or using the online calculator for your size tank.
Ca and especially Mg change much more slowly than Alk.I've only been dosing the recommended amount. I've noticed that the only thing that rises is the alk. the ca and MG stayed about the same.
That's normal.
ok I didn't know that. it does say to stop using afr and switch over to the balling A method if ca drops and alk rises. but I'll wait until my corals heal back up and alk stabilizes before I start dosing againCa and especially Mg change much more slowly than Alk.
That's normal.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,238
- Reaction score
- 63,591
ok I didn't know that. it does say to stop using afr and switch over to the balling A method if ca drops and alk rises. but I'll wait until my corals heal back up and alk stabilizes before I start dosing again
When Do Calcium and Alkalinity Demand Not Exactly Balance?
When Do Calcium and Alkalinity Demand Not Exactly Balance? By Randy Holmes-Farley Calcium and alkalinity are generally supplied to reef aquaria in order to offset losses caused by the formation of calcium carbonate. There are many ways to...
 www.reef2reef.com
www.reef2reef.com
Apparent Excess Demand for Alkalinity
One of the most common complaints of new aquarists is that their aquaria seem to need more alkalinity than their balanced additive system, such as limewater or All for Reef, is supplying. While there are reasons this may actually be the case over the long term (these will be detailed later in this article), frequently these aquarists are seeing a "chemical mirage" rather than a real excess demand for alkalinity.
One of the interesting features of seawater is that it contains a lot more calcium than alkalinity. By this, I mean that if all of the calcium in seawater (420 ppm) were to be precipitated as calcium carbonate, it would consume 59 dKH of alkalinity (nearly 10 times as much as is present in natural seawater). In a less drastic scenario, let's say that calcium carbonate is formed from aquarium water starting with an alkalinity of 8.4 dKH that it is allowed to drop to 5.6 dKH (a 33% drop). How much has the calcium declined? It is a surprise to many people to learn that the calcium would drop by only 20 ppm (5%). Consequently, many aquarists observe that their calcium levels are relatively stable (within their ability to reproducibly test it), but alkalinity can vary up and down substantially. This is exactly what would be expected, given that the aquarium already has such a large reservoir of calcium.
Therefore, the first "deviation" from the rule of calcium and alkalinity balance really isn't a deviation at all. If an aquarist is supplying a balanced additive to his aquarium, and calcium seems stable but alkalinity is declining, it may very well be that what is needed is more of the balanced additive, not just alkalinity. This scenario should be assumed as the most likely explanation for most aquarists who should look for more esoteric explanations for alkalinity decline only if calcium RISES substantially while alkalinity falls. Likewise, if alkalinity is rising and calcium seems stable when using a balanced calcium and alkalinity additive system, the most likely explanation is that too much of the additive system is being used.
The real imbalance effects described later in this article take effect slowly, and are manifested over weeks, months and years. This short term "chemical mirage" caused simply by the mathematics of calcium and alkalinity additions can be seen in a single addition. Any effect that develops rapidly over the course of a few days is almost certainly not a true demand imbalance.The following scenarios show what can happen to a reef aquarium whose dosage with a balanced additive system does not match its demand.
Table 1 shows what can happen when the dosing is inadequate. Alkalinity drops fairly rapidly. After one day, many aquarists might conclude that they need additional alkalinity, when in reality, they need more of both calcium and alkalinity to stabilize the system.
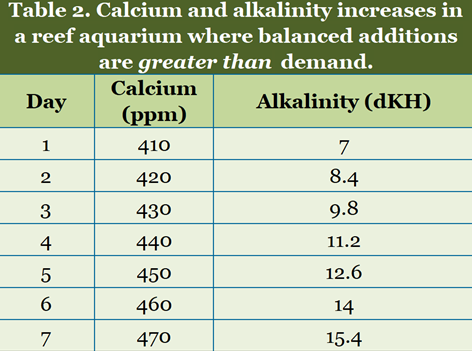
Table 2 shows what happens when too much of a balanced additive is added. After a day or two, many aquarists would conclude that alkalinity is rising too much, but that calcium is fairly stable. Again, what is needed is less of the balanced additive, not just less alkalinity.
I get it that i may have dosed too frequently, but I was only doing as instructed. I guess my next take would be to dose half of what's recommended next time
When Do Calcium and Alkalinity Demand Not Exactly Balance?
When Do Calcium and Alkalinity Demand Not Exactly Balance? By Randy Holmes-Farley Calcium and alkalinity are generally supplied to reef aquaria in order to offset losses caused by the formation of calcium carbonate. There are many ways to...www.reef2reef.com
Apparent Excess Demand for Alkalinity
One of the most common complaints of new aquarists is that their aquaria seem to need more alkalinity than their balanced additive system, such as limewater or All for Reef, is supplying. While there are reasons this may actually be the case over the long term (these will be detailed later in this article), frequently these aquarists are seeing a "chemical mirage" rather than a real excess demand for alkalinity.
One of the interesting features of seawater is that it contains a lot more calcium than alkalinity. By this, I mean that if all of the calcium in seawater (420 ppm) were to be precipitated as calcium carbonate, it would consume 59 dKH of alkalinity (nearly 10 times as much as is present in natural seawater). In a less drastic scenario, let's say that calcium carbonate is formed from aquarium water starting with an alkalinity of 8.4 dKH that it is allowed to drop to 5.6 dKH (a 33% drop). How much has the calcium declined? It is a surprise to many people to learn that the calcium would drop by only 20 ppm (5%). Consequently, many aquarists observe that their calcium levels are relatively stable (within their ability to reproducibly test it), but alkalinity can vary up and down substantially. This is exactly what would be expected, given that the aquarium already has such a large reservoir of calcium.
Therefore, the first "deviation" from the rule of calcium and alkalinity balance really isn't a deviation at all. If an aquarist is supplying a balanced additive to his aquarium, and calcium seems stable but alkalinity is declining, it may very well be that what is needed is more of the balanced additive, not just alkalinity. This scenario should be assumed as the most likely explanation for most aquarists who should look for more esoteric explanations for alkalinity decline only if calcium RISES substantially while alkalinity falls. Likewise, if alkalinity is rising and calcium seems stable when using a balanced calcium and alkalinity additive system, the most likely explanation is that too much of the additive system is being used.
The real imbalance effects described later in this article take effect slowly, and are manifested over weeks, months and years. This short term "chemical mirage" caused simply by the mathematics of calcium and alkalinity additions can be seen in a single addition. Any effect that develops rapidly over the course of a few days is almost certainly not a true demand imbalance.The following scenarios show what can happen to a reef aquarium whose dosage with a balanced additive system does not match its demand.
Table 1 shows what can happen when the dosing is inadequate. Alkalinity drops fairly rapidly. After one day, many aquarists might conclude that they need additional alkalinity, when in reality, they need more of both calcium and alkalinity to stabilize the system.
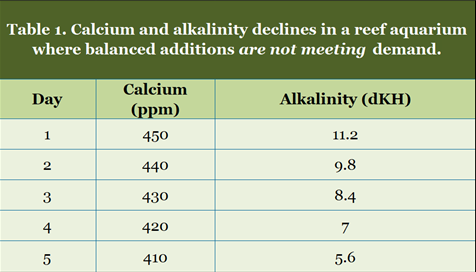
Table 2 shows what happens when too much of a balanced additive is added. After a day or two, many aquarists would conclude that alkalinity is rising too much, but that calcium is fairly stable. Again, what is needed is less of the balanced additive, not just less alkalinity.
I began dosing AFR 12/30/23, at that time my alk was 8.0 & cal 432, yesterday my alk was 12.1 & cal 434. My alk has been consistently climbing while my cal stays around the same number. How do I balance the two? I started with good numbers but can't seem to keep the alk down. I have a 25g tank & have been dosing 5ml daily. I do 10% wc every week using tropic marin salt.AFR is actually a little tiny bit heavy on calcium relative to alk, so that effect may just be the issues with calcium testing.
Thanks for any guidance you can provide!
AFR/ALK dose info i have.
2,5ml AFR in 20gallons raise alk by 2dkh

 www.reef2reef.com
www.reef2reef.com
I dose 3ml/day in 55gallons (i don't have a big consommation)
2,5ml AFR in 20gallons raise alk by 2dkh

Alk swingn at WC... need to test alk almost everyday (ocd) should i change salt
Hi, Reefcrystal... 1.026 and ~13dkh Tank, 1.025 dosing AFR. Alk~ 9dkh After a WC ... ALK 9,4.... WC ~10% /week TANK, zoa, monti. I just want stability, no precise target in mind... 8...9dkh its a good range i think. Each WC crank up my alk... (see graph) I find the alk consommation... Should i...
 www.reef2reef.com
www.reef2reef.com
I dose 3ml/day in 55gallons (i don't have a big consommation)
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,238
- Reaction score
- 63,591
I get it that i may have dosed too frequently, but I was only doing as instructed. I guess my next take would be to dose half of what's recommended next time
I’d back off on the dose until alk is stable where you want it.
Similar threads
- Replies
- 2
- Views
- 184
-
- Question
- Replies
- 14
- Views
- 202
- Replies
- 7
- Views
- 220