Follow up - i dose 5 ml there I always dose. First for the two probes that in the beginning not show any dip when I dose H2O2. Been in the aquarium for 1 month now (around)
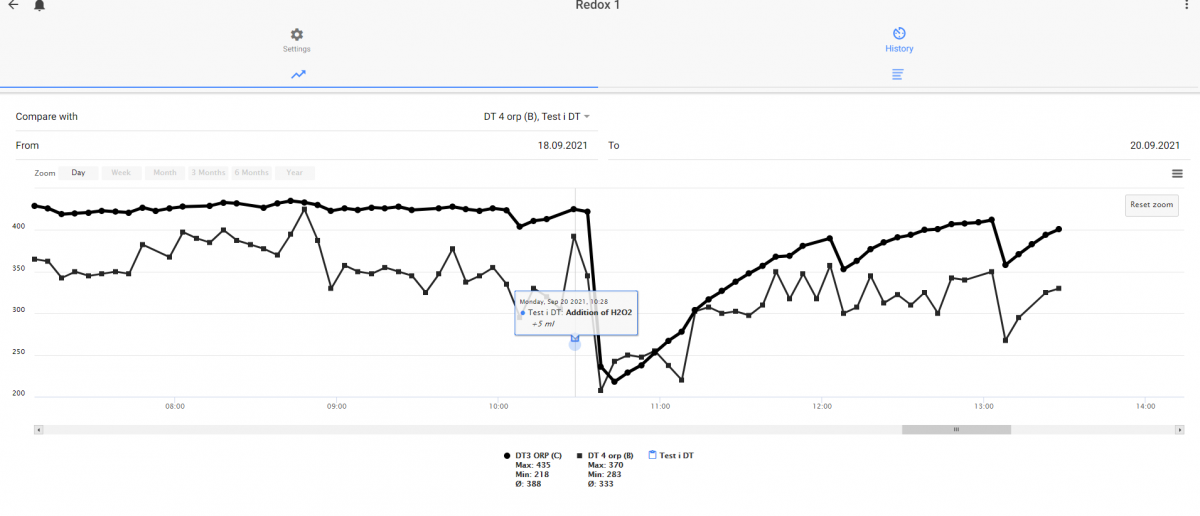
And Probe A - been in the aquarium for more than 2 years
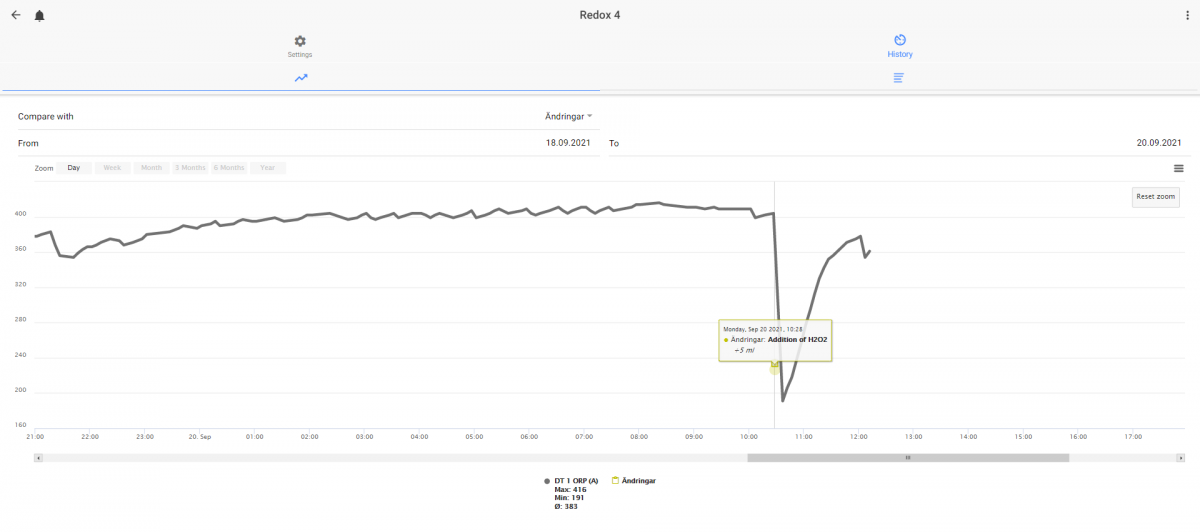
At least in my aquarium - the dip is depending on time in my aquarium
Sincerely Lasse
And Probe A - been in the aquarium for more than 2 years
At least in my aquarium - the dip is depending on time in my aquarium
Sincerely Lasse
Last edited:


















