- Joined
- Aug 24, 2016
- Messages
- 1,498
- Reaction score
- 2,292
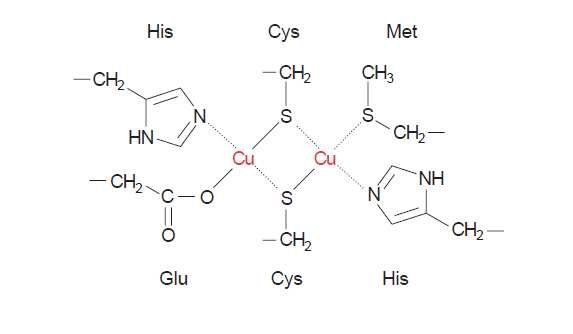
Ok, but in practical alginate application monovalent cations like sodium and potassium form more or less viscous liquids with alginic acid while divalent cations like calcium and magnesium form gels with alginic acid. In theory this is explained with the "egg box model", where the divalent ions lay between zig-zag-chains of alginic acid like eggs in an egg box.Both Cu+ and Cu++ can bind strongly to organics.