- Joined
- May 22, 2016
- Messages
- 6,527
- Reaction score
- 10,061
This is interesting, but includes a lot of speculation, which I've tried to make clear.
A. Test Method: Possibly Measuring a Polyquat Algaecide in Reef Tank Water (tracking Vibrant dose)
(almost. Kinda, but not really.)
B. Hypothesis: The algaecide in question is a cationic polymer, which combines with anionic surfactant, SDS (sodium dodecyl sulfate) to produce structures that are large enough to scatter light and thus the cloudy water from that process can be measured by a hanna checker. Detection by this method can be done in distilled water at much higher concentrations, but it is not sensitive enough to detect a recommended dose (1 mL product / 10 Gallons) in new saltwater. But in my reef tank water, this method does form easily quantifiable cloudiness in response to addition of the product, suggesting an interaction with organics / particulates etc in reef water that makes the measurement more sensitive. (I have no idea if it will work in anyone else's water or not.)
C. Method: (I'm not going to do a bunch of tweaking on this, so I'll leave it here for reference and others can run with it or not.)
I'm using the hanna ULR P meter hi736, and 10% SDS and below is the method as I have been using it. The product being added to the tank is Vibrant.
- Take 10 mL tank water, add to a hanna cuvette (use that as blank, C1)
- add 0.50mL of 10% SDS to the cuvette
- cap and invert 10x to mix (do not shake, avoid bubbles)
- start 3 minute countdown
- gently invert one last time ~30-40 sec before measurement (likely unnecessary).
- measure in checker
D. Here's some data showing what applying this method to Vibrant doses in my tank generates.
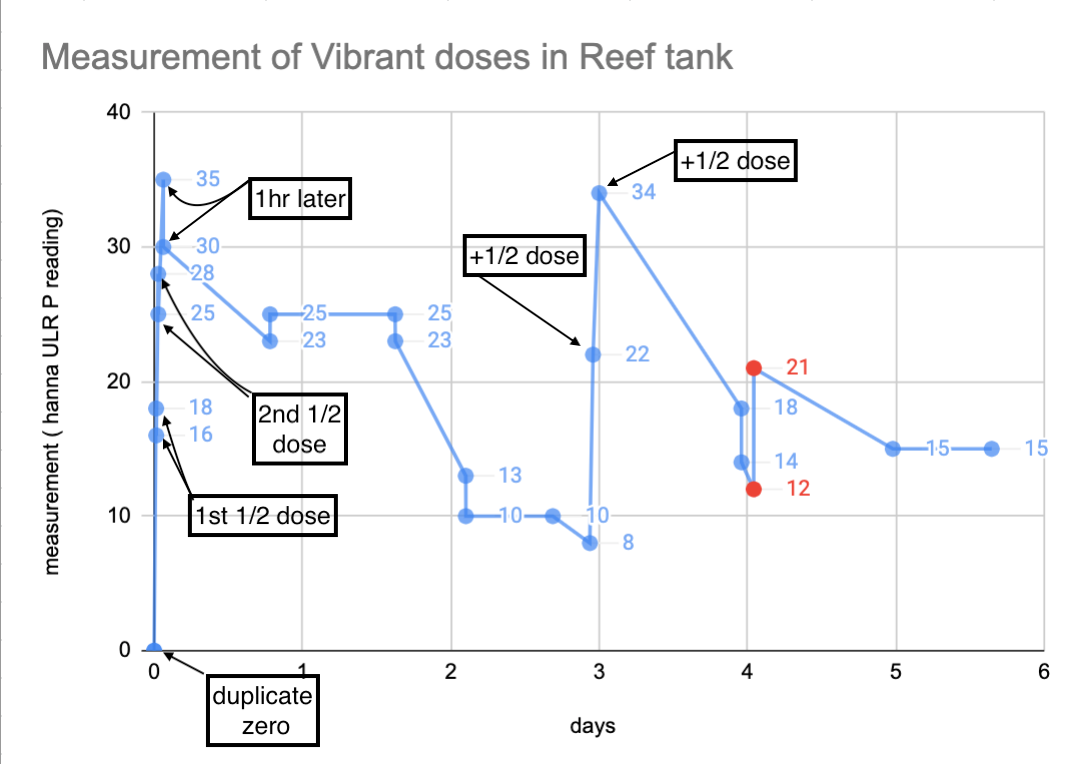
My tank has a history of Vibrant and Algaefix use, both of which are detected by this method. Neither product had been added to the tank in 2+ months.
Prior to the additions shown on this chart, my tank water gave consistent zeros using this measurement. I added 1/2 of a recommended dose (recommended = 1mL / 10 Gal) of Vibrant and measured ~30min later. Then repeated with another 1/2 dose and 30min wait.
After 3 full days I added another recommended dose split in half again and measured the same way. I did duplicates for all measurements for the first 2 days. (I'll explain the red dots in a later post.)
My tank runs a skimmer 24/7 and some GFO.
It's worth pointing out that this is not a direct measurement of the polyquat itself, because doing so in distilled water or fresh mixed Instant Ocean does not have near this sensitivity. My speculation is that the chemical interacts with organics in my tank water, so it's probably more accurate to say I'm measuring Vibrant-associated-material, after it interacts with my tank water. This Vibrant associated material seems to deplete from the water mostly (but not totally) over 3 days. If your tank water is like mine, the method ought to work similarly. If you do a bunch of water changes (I don't) then it probably won't. So this isn't true quantification, but it may be possible to track the relative rates of removal of a dose of this material from the water in systems other than mine. But if the amount of organics in your water dramatically decreases, then this method of detection would become less responsive to Vibrant.
(I'll post background theory for those interested in reading more, and more observations later.)
A. Test Method: Possibly Measuring a Polyquat Algaecide in Reef Tank Water (tracking Vibrant dose)
(almost. Kinda, but not really.)
B. Hypothesis: The algaecide in question is a cationic polymer, which combines with anionic surfactant, SDS (sodium dodecyl sulfate) to produce structures that are large enough to scatter light and thus the cloudy water from that process can be measured by a hanna checker. Detection by this method can be done in distilled water at much higher concentrations, but it is not sensitive enough to detect a recommended dose (1 mL product / 10 Gallons) in new saltwater. But in my reef tank water, this method does form easily quantifiable cloudiness in response to addition of the product, suggesting an interaction with organics / particulates etc in reef water that makes the measurement more sensitive. (I have no idea if it will work in anyone else's water or not.)
C. Method: (I'm not going to do a bunch of tweaking on this, so I'll leave it here for reference and others can run with it or not.)
I'm using the hanna ULR P meter hi736, and 10% SDS and below is the method as I have been using it. The product being added to the tank is Vibrant.
- Take 10 mL tank water, add to a hanna cuvette (use that as blank, C1)
- add 0.50mL of 10% SDS to the cuvette
- cap and invert 10x to mix (do not shake, avoid bubbles)
- start 3 minute countdown
- gently invert one last time ~30-40 sec before measurement (likely unnecessary).
- measure in checker
D. Here's some data showing what applying this method to Vibrant doses in my tank generates.
My tank has a history of Vibrant and Algaefix use, both of which are detected by this method. Neither product had been added to the tank in 2+ months.
Prior to the additions shown on this chart, my tank water gave consistent zeros using this measurement. I added 1/2 of a recommended dose (recommended = 1mL / 10 Gal) of Vibrant and measured ~30min later. Then repeated with another 1/2 dose and 30min wait.
After 3 full days I added another recommended dose split in half again and measured the same way. I did duplicates for all measurements for the first 2 days. (I'll explain the red dots in a later post.)
My tank runs a skimmer 24/7 and some GFO.
It's worth pointing out that this is not a direct measurement of the polyquat itself, because doing so in distilled water or fresh mixed Instant Ocean does not have near this sensitivity. My speculation is that the chemical interacts with organics in my tank water, so it's probably more accurate to say I'm measuring Vibrant-associated-material, after it interacts with my tank water. This Vibrant associated material seems to deplete from the water mostly (but not totally) over 3 days. If your tank water is like mine, the method ought to work similarly. If you do a bunch of water changes (I don't) then it probably won't. So this isn't true quantification, but it may be possible to track the relative rates of removal of a dose of this material from the water in systems other than mine. But if the amount of organics in your water dramatically decreases, then this method of detection would become less responsive to Vibrant.
(I'll post background theory for those interested in reading more, and more observations later.)




















