- Joined
- Jan 1, 2019
- Messages
- 120
- Reaction score
- 213
How can we make sure we are getting accurate results when testing? Knowing what can cause erroneous results helps as does developing good testing habits.
A bit about me, I am a plant operator and the QA person in a global chemical additive company. We have a pretty strict policy on how to take a sample and how we maintain the testing equipment. When I sat back and thought what if I applied this to my tank testing would it make any difference. Well to my joy I started seeing more consistent results in my test results, they were no longer all over the place. So here is my method of sampling and testing.
First, make sure your kits/reagents are not expired.
Knowing how to read an expiration date can save lots of headaches. Some kits are strait forward and show month and year of expiration others not so much. API shows a two digit month and a four digit year such as 06/2020 meaning the kit expires on June in 2020. A redsea kit may have an expiration number like 0620 for the same month and year.
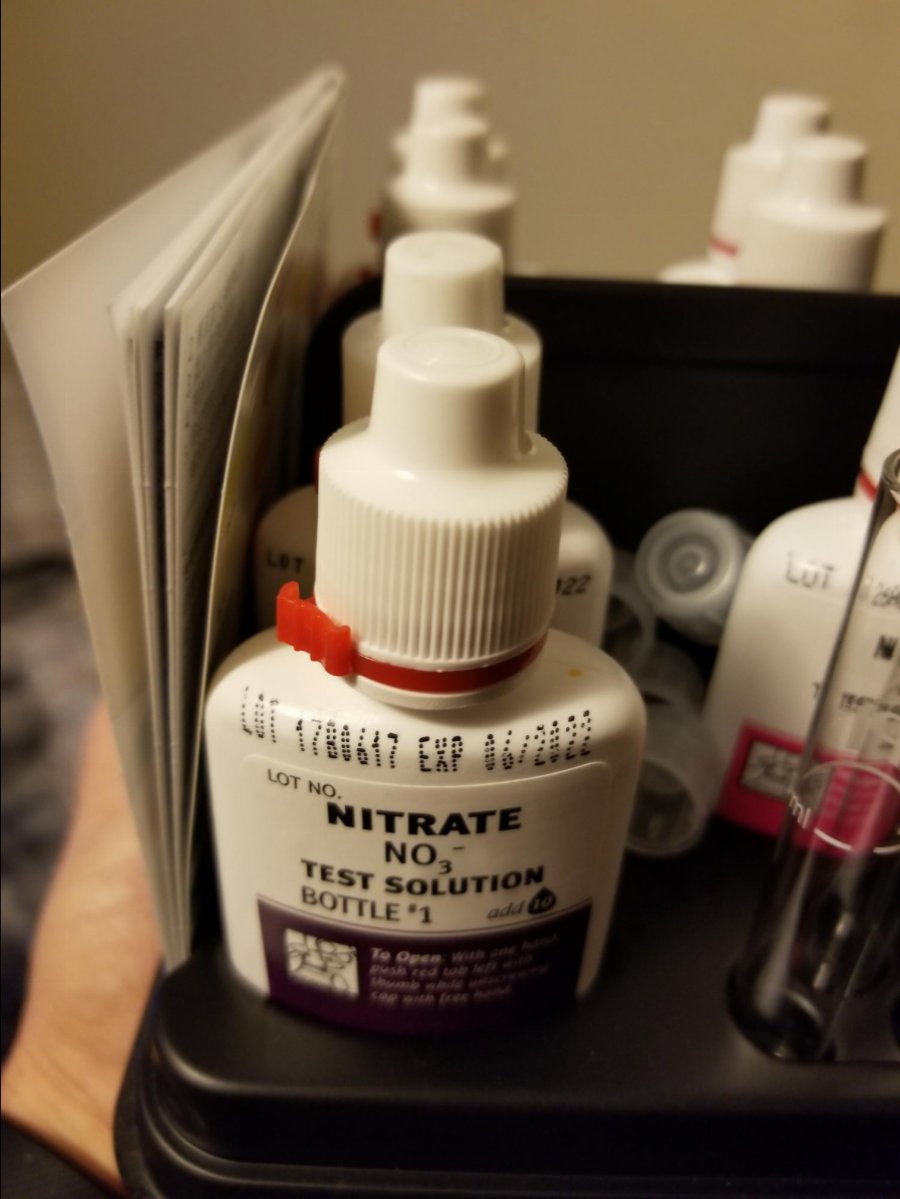
Second, our equipment.
Having clean equipment in good working order (lab grade is always best) is essential for getting consistent testing.

Rinsing and drying syringes, vials and pipettes has a great impact on test results. Using distilled or RO water to rinse before and after testing and lint free or micro fiber cloth for drying helps keep cuvettes and vials in tip top condition. Occasionally you may need to clean your glass items in a citric or vinegar bath to remove residue.


How we take a sample is pretty important also. Rinsing your syringe, pipette, tube or vial with the water to be tested by filling and dumping (down the drain not back into the tank) three times. This seems like a lot but it will most likely remove any residue or contaminates like salt crust left over from the last use that could effect your results.
Time of day has an impact on results. Your PH can change during the day according to your photo period, by testing around the same time every day we can ensure we are getting accurate results that should be similar. Additives can effect results too. Read as much as you can about what you are putting in your tank and try not to test right after adding something unless required to according to the additive.
How the testing solutions/reagents are added is very important. A pipet or dropper bottle held at a angle can deliver a slightly different size drop then one if the pipette/ dropper bottle is held vertically.

Holding vertically is the normal standard for using a dropper bottle.

For dry powders I will get a scoop of reagent using the kits supplied scoop and then level the scoop by drawing the flat edge of index card across the top. (yes this is a large spoon but the small scoops in a kit are hard to photograph doing this)

So now that I have covered that, here is how I typically test. Normally I perform my tank tests at 4pm to 5pm before I leave for the day. I gather a single large sample in a suitable container. I have access to lab grad beakers and sample cups and opt for a sample cup and syringe. Both are rinsed with distilled water before use.

I rinse the clean sample cup with tank water three times and then fill the cup one more time, this will be the water I will be testing. From this point on this water will be considered "unsuitable for tank use" in the idea that it is good for testing but I should not return it to the tank as it may inadvertently get something in it that I do not want to introduce into my tank. From this point on its all a matter of following the test kits instructions. When the test is complete I rinse the testing tube or vial immediately with tap water a minimum of three times followed by distilled or RO water and dry them to keep the glass in as pristine condition as possible. Reagents sitting in a test container can etch and discolor it.
Testing for salinity I use a refractometer. Mine instructs that calibration should be done with distilled water with the reading being zero. Since I need to rinse it off after use I actually calibrate when I am finished testing. First I use the pipette that came with it and gather my sample by drawing up water from the tank three times and squirting it back out each time, This again removes any salt residue that may have remained in it from last use and cleaning. I place the recommended three drops on the refractometer and take my reading. Next I rinse the refractometer and cover with distilled water toughly, close the cover and take a reading. If it is still in calibration I should get a zero reading. Now I can dry off everything and put it back in its storage case. Doing it this way I still know it is in calibration and saved a bit on the distilled water and an extra step.

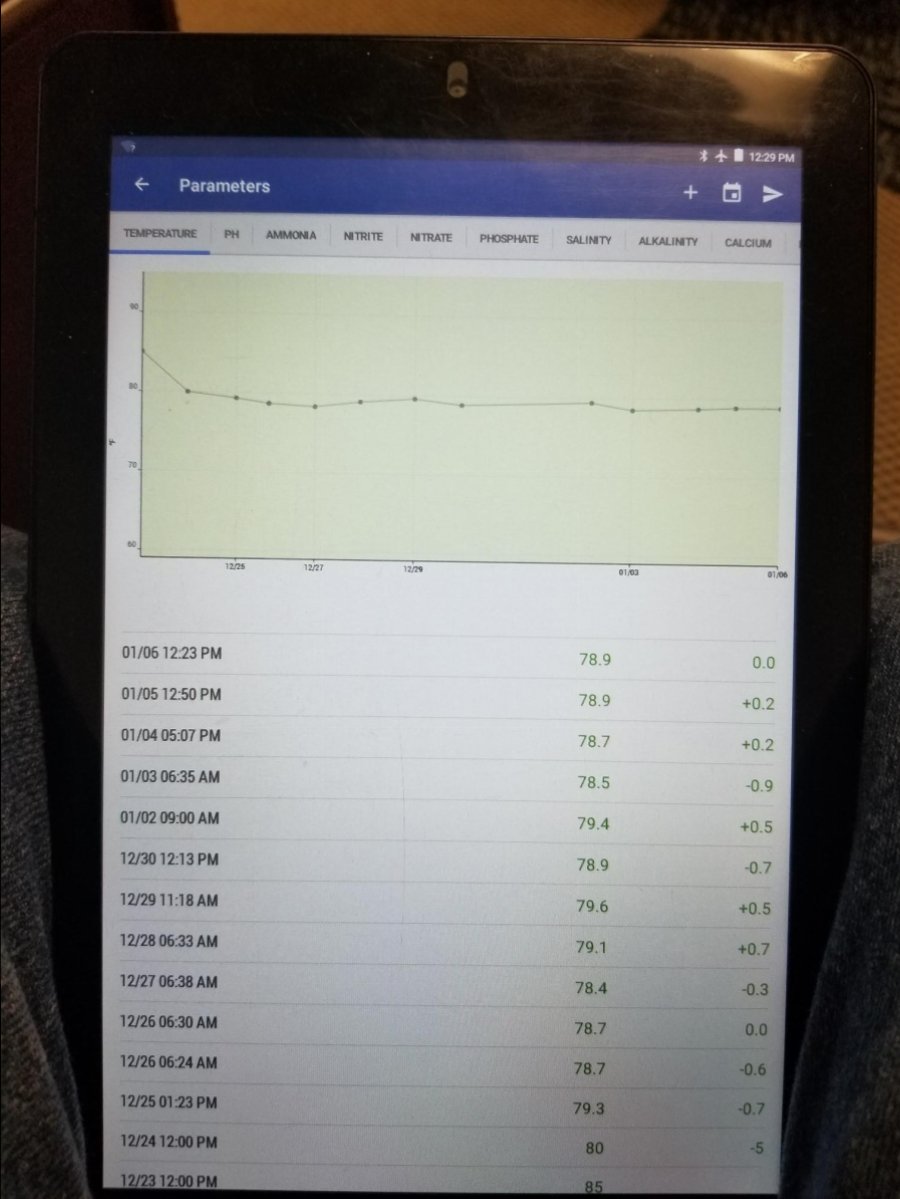
Recording results in a manner that can be displayed as a graph is a great way to see at a glance if there is a trend or problem starting up. Here you can see me dialing in my heater to the desired temperature over a weeks time during set up.
So by keeping everything as clean as possible, make sure to get the best possible representative sample of tank water along with good testing technique we can be assured our results will be more accurate of what is going on in our tanks.
A bit about me, I am a plant operator and the QA person in a global chemical additive company. We have a pretty strict policy on how to take a sample and how we maintain the testing equipment. When I sat back and thought what if I applied this to my tank testing would it make any difference. Well to my joy I started seeing more consistent results in my test results, they were no longer all over the place. So here is my method of sampling and testing.
First, make sure your kits/reagents are not expired.
Knowing how to read an expiration date can save lots of headaches. Some kits are strait forward and show month and year of expiration others not so much. API shows a two digit month and a four digit year such as 06/2020 meaning the kit expires on June in 2020. A redsea kit may have an expiration number like 0620 for the same month and year.
Second, our equipment.
Having clean equipment in good working order (lab grade is always best) is essential for getting consistent testing.
Rinsing and drying syringes, vials and pipettes has a great impact on test results. Using distilled or RO water to rinse before and after testing and lint free or micro fiber cloth for drying helps keep cuvettes and vials in tip top condition. Occasionally you may need to clean your glass items in a citric or vinegar bath to remove residue.
How we take a sample is pretty important also. Rinsing your syringe, pipette, tube or vial with the water to be tested by filling and dumping (down the drain not back into the tank) three times. This seems like a lot but it will most likely remove any residue or contaminates like salt crust left over from the last use that could effect your results.
Time of day has an impact on results. Your PH can change during the day according to your photo period, by testing around the same time every day we can ensure we are getting accurate results that should be similar. Additives can effect results too. Read as much as you can about what you are putting in your tank and try not to test right after adding something unless required to according to the additive.
How the testing solutions/reagents are added is very important. A pipet or dropper bottle held at a angle can deliver a slightly different size drop then one if the pipette/ dropper bottle is held vertically.
Holding vertically is the normal standard for using a dropper bottle.
For dry powders I will get a scoop of reagent using the kits supplied scoop and then level the scoop by drawing the flat edge of index card across the top. (yes this is a large spoon but the small scoops in a kit are hard to photograph doing this)
So now that I have covered that, here is how I typically test. Normally I perform my tank tests at 4pm to 5pm before I leave for the day. I gather a single large sample in a suitable container. I have access to lab grad beakers and sample cups and opt for a sample cup and syringe. Both are rinsed with distilled water before use.
I rinse the clean sample cup with tank water three times and then fill the cup one more time, this will be the water I will be testing. From this point on this water will be considered "unsuitable for tank use" in the idea that it is good for testing but I should not return it to the tank as it may inadvertently get something in it that I do not want to introduce into my tank. From this point on its all a matter of following the test kits instructions. When the test is complete I rinse the testing tube or vial immediately with tap water a minimum of three times followed by distilled or RO water and dry them to keep the glass in as pristine condition as possible. Reagents sitting in a test container can etch and discolor it.
Testing for salinity I use a refractometer. Mine instructs that calibration should be done with distilled water with the reading being zero. Since I need to rinse it off after use I actually calibrate when I am finished testing. First I use the pipette that came with it and gather my sample by drawing up water from the tank three times and squirting it back out each time, This again removes any salt residue that may have remained in it from last use and cleaning. I place the recommended three drops on the refractometer and take my reading. Next I rinse the refractometer and cover with distilled water toughly, close the cover and take a reading. If it is still in calibration I should get a zero reading. Now I can dry off everything and put it back in its storage case. Doing it this way I still know it is in calibration and saved a bit on the distilled water and an extra step.
Recording results in a manner that can be displayed as a graph is a great way to see at a glance if there is a trend or problem starting up. Here you can see me dialing in my heater to the desired temperature over a weeks time during set up.
So by keeping everything as clean as possible, make sure to get the best possible representative sample of tank water along with good testing technique we can be assured our results will be more accurate of what is going on in our tanks.

















