sixty_reefer
5000 Club Member
View BadgesArticle Contributor
UK Reef Club Member
Hospitality Award
R2R Research
The ability of home test kits to extract total nitrogen and phosphorus from organics is what I’m finding interesting
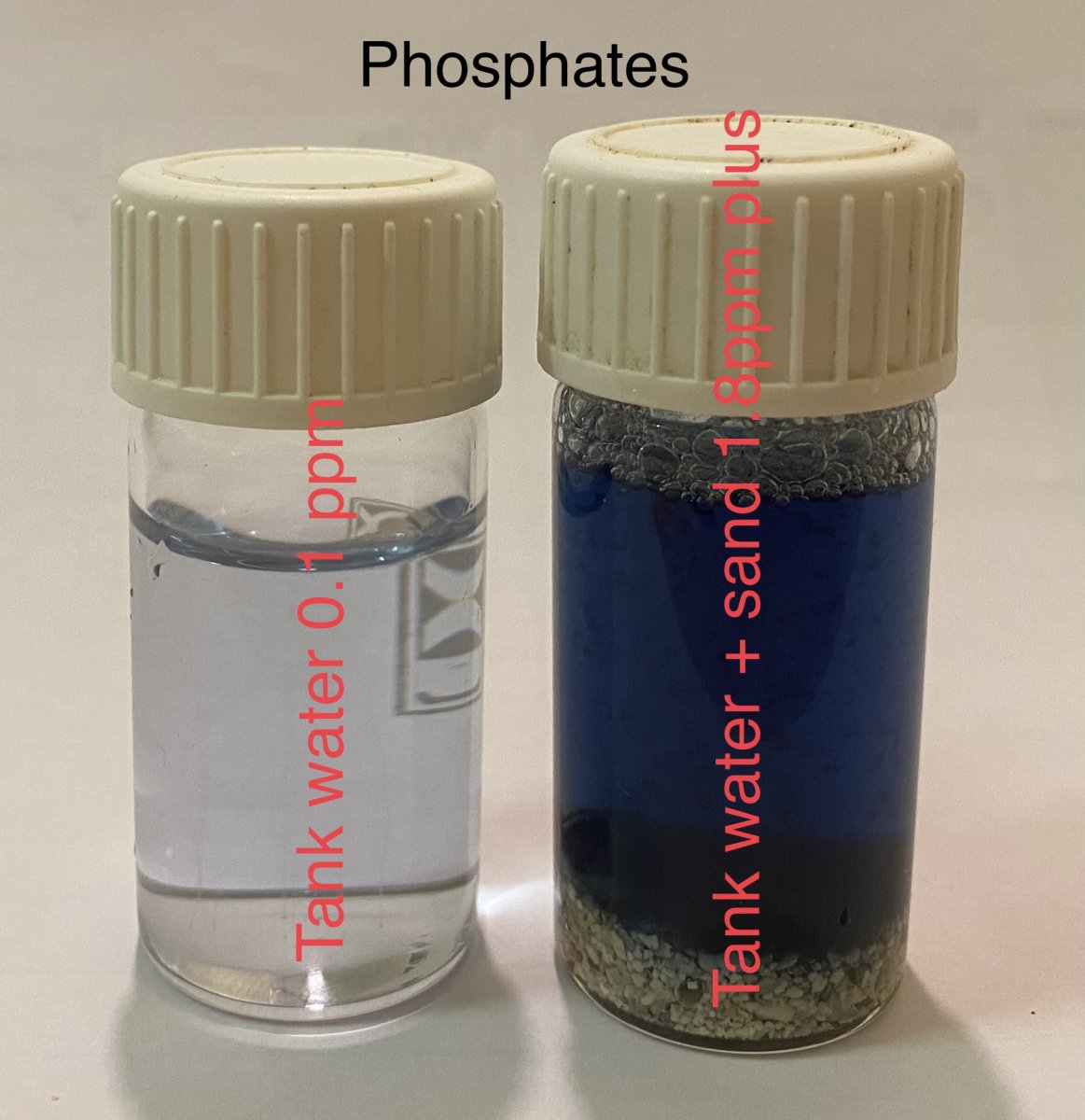
total phosphates from sand and organics on the right, total organics and inorganic from the water column on the left
total phosphates from the organics trapped on the sand only

the question here is if I am able to filter organics from the water column, would I have less than 0.1 ppm phosphates available in my tank. Making it a DIY test to separate organic phosphates from inorganic phosphate. What kind of filter would I need to remove organics from the water column @Randy Holmes-Farley?
If my assumption is correct we should be able to test for organic and inorganic N and P at home by applying a specialised filter.
Calculate the total phosphates and reduce it from the phosphates tested with a special filter that should test for inorganic only.
what this thread may be starting to show is that organics in the water column can affect the accuracy of home test kits, if there is many micro algaes floating on the water column, like the ones we see on our glass they may be affecting the results greatly as our test kits have the ability to break them down and show them as phosphates or nitrates. The development of a filter for our syringe should aid to remove this variables and differentiate organic phosphates from inorganic phosphates.
total phosphates from sand and organics on the right, total organics and inorganic from the water column on the left
total phosphates from the organics trapped on the sand only
the question here is if I am able to filter organics from the water column, would I have less than 0.1 ppm phosphates available in my tank. Making it a DIY test to separate organic phosphates from inorganic phosphate. What kind of filter would I need to remove organics from the water column @Randy Holmes-Farley?
If my assumption is correct we should be able to test for organic and inorganic N and P at home by applying a specialised filter.
Calculate the total phosphates and reduce it from the phosphates tested with a special filter that should test for inorganic only.
what this thread may be starting to show is that organics in the water column can affect the accuracy of home test kits, if there is many micro algaes floating on the water column, like the ones we see on our glass they may be affecting the results greatly as our test kits have the ability to break them down and show them as phosphates or nitrates. The development of a filter for our syringe should aid to remove this variables and differentiate organic phosphates from inorganic phosphates.
Last edited:

















