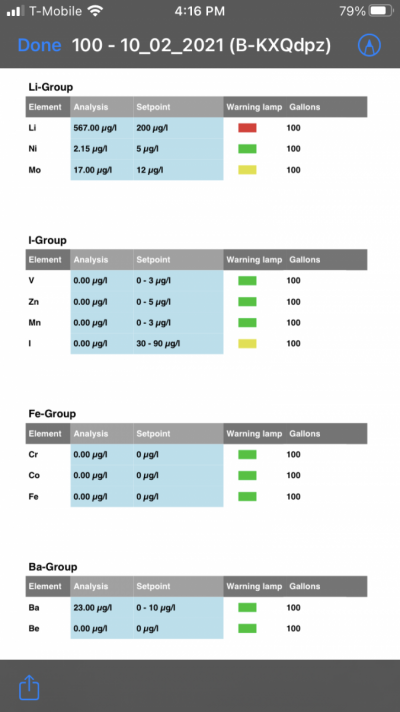
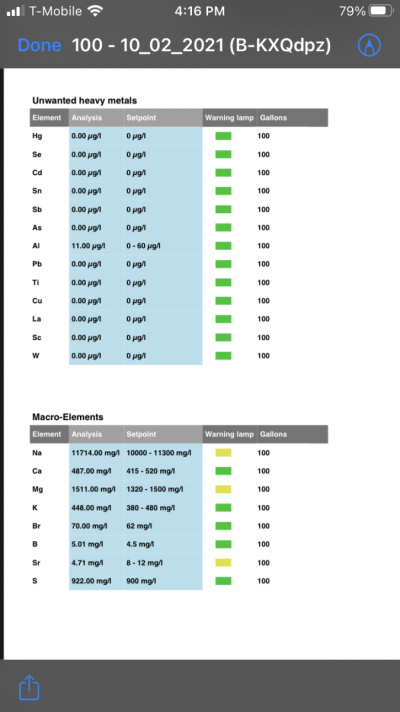
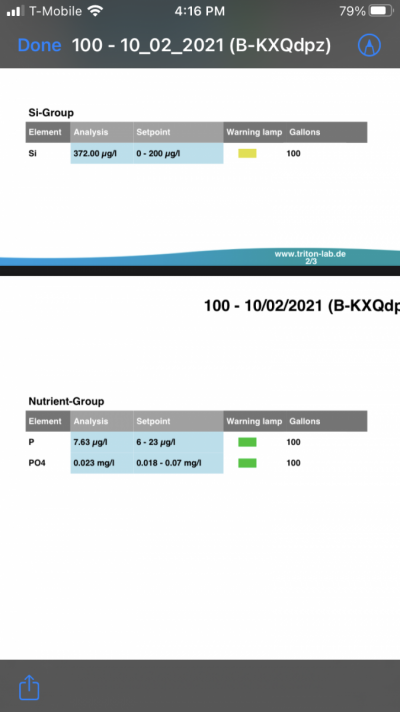
- Joined
- Jul 3, 2019
- Messages
- 86
- Reaction score
- 83
I'm trying to decide which 2 (or 3, or 4) part system I want to use with my reef, and am currently comparing Tropic Marin (TM) vs Bulk Reef Supply (BRS) Pharma parts A & B.
Here are some observations and related questions.
Tropic Marin Part A uses Calcium Chloride Dihydrate, while BRS is just Calcium Chloride. Reading the directions / using calculators, straight Calcium Chloride seems to raise the dKH by roughly twice the amount as Calcium Chloride Dihydrate.
<Q1>Is that correct? Calcium Chloride Dihydrate adds ~1/2 the carbonate hardness that straight Calcium Chloride does?
<Q2>What, if any, is the advantage of Calcium Chloride Dihydrate over Calcium Chloride?
Tropic Marin part B is a mixture of Sodium Carbonate and Sodium Bicarbonate. It takes 286g vs 302g of BRS sodium bi-carbonate to make a gallon of solution, and the dkH bump from a 30ml dose of each solution is roughly equivalent (raises 2.2dKH)
<Q>Why the mixture in Tropic Marine Part B of sodium carbonate/bicarbonate?
I did a bunch of math accounting for the different strengths and amounts needed (was a 'B' student when it came to Algebra so this may be wrong!). My calculations show BRS Calcium Chloride is approximately 42% of the cost of Tropic Marine Part A for the equivalent dKH boost.
<Q>Any reason to go with Tropic Marin part A & B and pay the premium instead of BRS pharma?
<Q>I'm guessing that the Tropic Marin formula's advantage is that I can dose equal parts A&B and everything stays balanced? Where as with BRS sodium bicarbonate (or sodium carbonate) / calcium chloride mixes, there is not a simple 1:1 between parts A&B and I have to dose different amounts to keep my ionic balance? (not considering TM part C which is another topic).
<Q>How does BRS Magnesium factor in the equation?
I've gone through two boxes of TM Part B (Sodium Carbonate/Bicarbonate) and both were clumped up really bad, and required lots of smashing and powder in the air to get something scoop-able. I don't mind paying a premium for TM if there are other advantages, and hopefully future TM part B boxes will not be clumped?
So confused! This makes me want to re-take high school and college chemistry.
-Justin
Here are some observations and related questions.
Tropic Marin Part A uses Calcium Chloride Dihydrate, while BRS is just Calcium Chloride. Reading the directions / using calculators, straight Calcium Chloride seems to raise the dKH by roughly twice the amount as Calcium Chloride Dihydrate.
<Q1>Is that correct? Calcium Chloride Dihydrate adds ~1/2 the carbonate hardness that straight Calcium Chloride does?
<Q2>What, if any, is the advantage of Calcium Chloride Dihydrate over Calcium Chloride?
Tropic Marin part B is a mixture of Sodium Carbonate and Sodium Bicarbonate. It takes 286g vs 302g of BRS sodium bi-carbonate to make a gallon of solution, and the dkH bump from a 30ml dose of each solution is roughly equivalent (raises 2.2dKH)
<Q>Why the mixture in Tropic Marine Part B of sodium carbonate/bicarbonate?
I did a bunch of math accounting for the different strengths and amounts needed (was a 'B' student when it came to Algebra so this may be wrong!). My calculations show BRS Calcium Chloride is approximately 42% of the cost of Tropic Marine Part A for the equivalent dKH boost.
<Q>Any reason to go with Tropic Marin part A & B and pay the premium instead of BRS pharma?
<Q>I'm guessing that the Tropic Marin formula's advantage is that I can dose equal parts A&B and everything stays balanced? Where as with BRS sodium bicarbonate (or sodium carbonate) / calcium chloride mixes, there is not a simple 1:1 between parts A&B and I have to dose different amounts to keep my ionic balance? (not considering TM part C which is another topic).
<Q>How does BRS Magnesium factor in the equation?
I've gone through two boxes of TM Part B (Sodium Carbonate/Bicarbonate) and both were clumped up really bad, and required lots of smashing and powder in the air to get something scoop-able. I don't mind paying a premium for TM if there are other advantages, and hopefully future TM part B boxes will not be clumped?
So confused! This makes me want to re-take high school and college chemistry.
-Justin