Understanding Colorimetry and Your Hanna Checker® HC
By: Kevin Costa, Hanna Instruments
Intro
Maintaining water quality and critical parameters is the single most important aspect of having a successful aquarium. Knowing your water's parameters is usually the first troubleshooting step to any health issue arising in aquatic life and is vital to maintaining sensitive fish, coral, or other ornamentals. As aquarium technologies develop, so do the tools we use to test our water. Hobbyists are no longer constrained to simple chemical test kits, but now have a multitude of options ranging in scientific complexity to analyze their reef tank’s water chemistry.
One tool used widely by reefers is our Hanna Checker. The Hanna Checker® HC Series has been a valuable resource for reefkeepers to accurately test their aquaria. The HC stands for handheld colorimeter, because they utilize the principles of colorimetry for testing. The Checker HCs bridge the gap between simple chemical test kits and professional instrumentation. By using a fixed light source, proven chemical method, and dedication to a single parameter, we have provided an affordable, accurate, and easy to use testing system that rivals other instruments that cost thousands of dollars. But have you ever wondered what actually is going on inside your Checker? Colorimeters are commonly used in a variety of applications and measure absorbance from specific wavelengths of light in conjunction with certain reagents. Learn more below to understand how your Checker works, the principles of colorimetry, and other photometric instruments.
Light and Color
Before entering into colorimetry, it’s important to understand the relationship between light and color. In simple terms, colors are dependent on light. When white light shines on an object, it may be reflected, absorbed, and/or transmitted. Glass transmits most of the light that comes into contact with it, thus it appears colorless. Snow reflects all of the light and appears white. A black cloth absorbs all light, so it appears black. A red piece of paper reflects red light better than it reflects other colors.
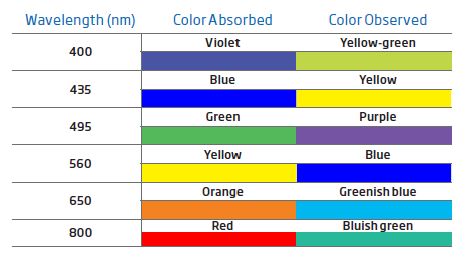
Objects appear colored because their chemical structure absorbs certain wavelengths of light and reflects others. The wavelengths of visible light that are reflected are what we see.
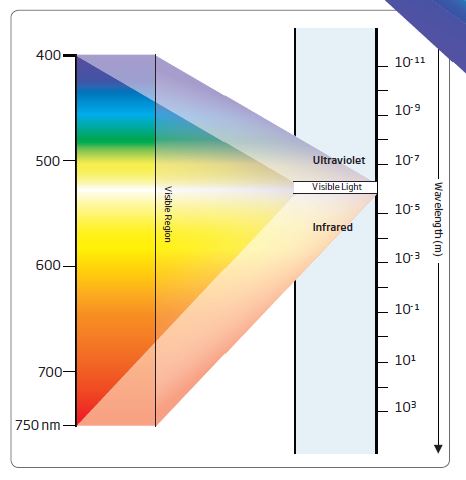
When discussing light, we are usually referring to white (visible) light. When white light passes through a prism (a triangular transparent object) the colors that make up white light disperse into seven bands of color. These bands of color are called a spectrum. Seven primary colors make up white light: red, orange, yellow, green, blue, indigo, and violet.
Suppose we shine a beam of white light at a substance that absorbs blue light. Since the blue component of the white light gets absorbed by the substance, the light that is transmitted is mostly yellow, the complementary color of blue. This yellow light reaches our eyes, and we “see” the substance as a yellow colored substance.
The color variation of a system that undergoes a change in concentration of some component is the basis of colorimetric analysis.
Colorimetry
Colorimetry is simply the measurement of color; it is the determination of the concentration of a substance by measurement of the relative absorption of light with respect to a known concentration of the substance. In visual colorimetry, natural or artificial white light is generally used as a light source and determinations are usually made with a instrument called a colorimeter, or color comparator. When the eye is replaced by a photoelectric cell, the instrument is termed a photoelectric colorimeter.
A colorimetric analysis is based on the principle that many substances react with each other and form a color with an intensity that correlates to the concentration of the substance to be measured. When a substance is exposed to a beam of light of intensity (I₀) a portion of the radiation is absorbed by the substance’s molecules and a radiation of intensity (I) is emitted. This difference in intensity is used for the colorimetric determination.
The quantity of radiation absorbed is given by the Beer-Lambert Law:

Absorbance is also given by: A= ελ • C • l where:
A is a dimensionless number
ελ the proportionality constant, is called the molar extinction coefficient or molar absorptivity; it’s a constant for a given substance, provided the temperature and wavelength are constant [L/(mol•cm)]
C concentration of the substance (mol/liter)
l optical distance light travels through sample (cm)
Therefore, the concentration (C) can be calculated from the absorbance of the substance determined by the emitted radiation (I), as the other factors are known.
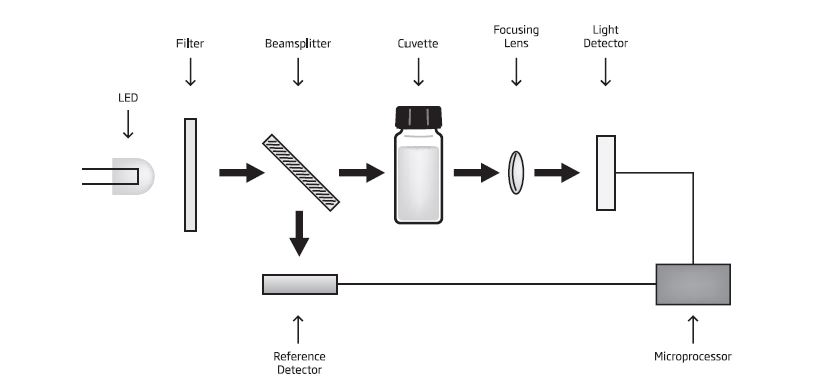
A typical block diagram of a photometer is shown below:
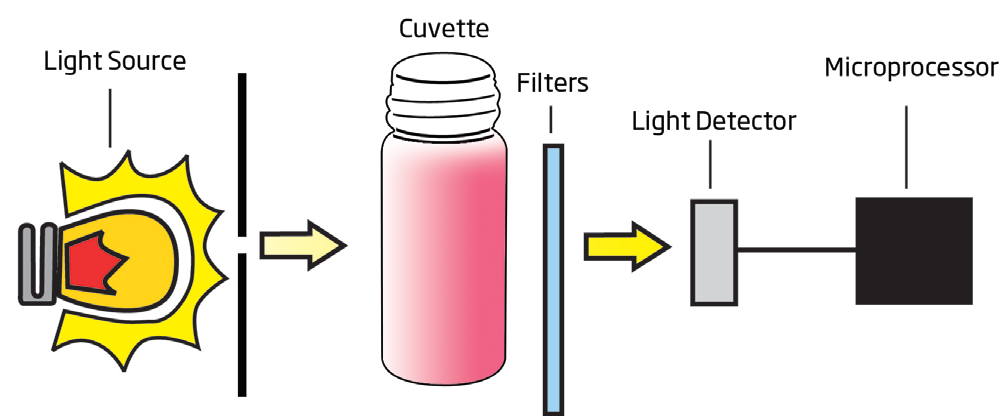
Sources of light used by Hanna colorimeters:
-Tungsten lamp: an incandescent lamp having a tungsten filament
-LED light emitting diode
The optical distance is measured by the dimension of the cuvette containing the sample. The photoelectric cell collects the radiation (I) emitted by the sample and converts it into an electric current, producing a potential in the millivolt (mV) range. The microprocessor uses this potential to convert the incoming value into the desired measuring unit and display it on the LCD. In fact, the preparation of the solution to be measured occurs under known conditions, which are programmed into the meter’s microprocessor in the form of a calibration curve. This curve is used as a reference for each measurement. It’s then possible to determine unknown concentrations of a sample by using a colorimetric reaction and the mV signal separated by a sensor in relation to the emitted intensity (I) (the color of the sample). By employing the calibration curve, you can determine the concentration of the sample that corresponds to the mV value. Supposing that for one chemical substance there is a maximum absorbance at 610 nm. I represents the light intensity after passing through a reacted sample while (I₀) equals the light intensity after passing an unreacted sample. As light passes through a solution, some is absorbed while some is transmitted through a solution. The difference between I₀ and I is used to determine the concentration. It’s important to also establish a zero readings before adding reagent to the sample to compensate for any color present prior to reagent being added.
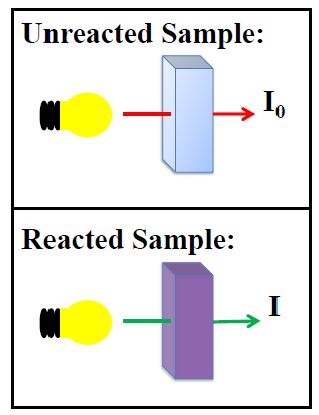
Absorbance also depends upon the concentration of the absorbing compound in the path length of the light through the cuvette. Thus differences in the path length can effect absorbance. Since cuvettes are not uniformly round, this can have an impact on path length. Indexing the cuvettes in the same position from C1 to C2 helps make sure the path length is the same and creates minimal variation in results from C1 to C2. Furthermore, we also want to keep the cuvettes free of scratches, fingerprints, smudges, or any imperfections which can impede the light, and have an impact on results. With the following graphs, you have one example of how the colorimeters are working to determine concentration:
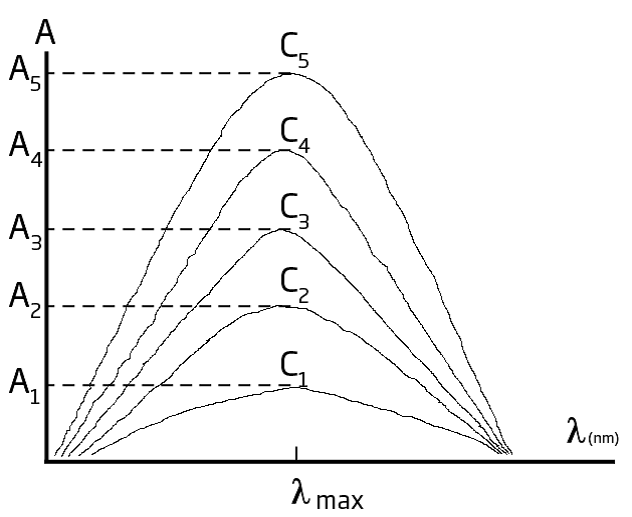
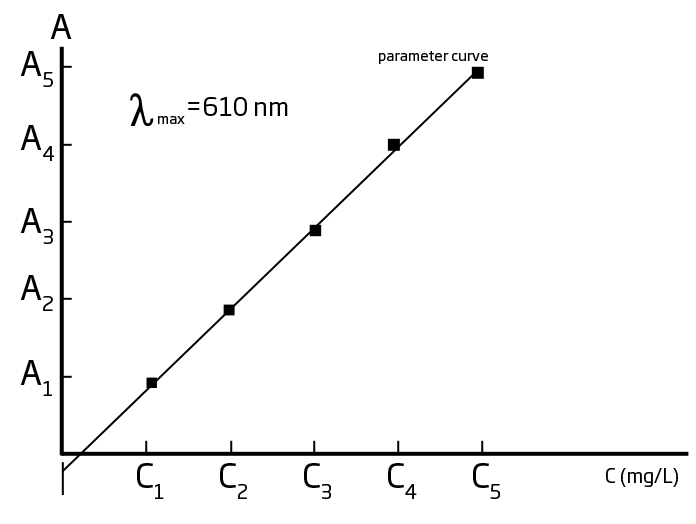
One example of an early colorimetric analysis is Nessler’s method for ammonia, which was first proposed in 1856. Nessler found that adding an alkaline solution of HgI2 and KI to a dilute solution of ammonia produced a yellow to reddish brown colloid, with the color intensity proportional to the concentration of ammonia present.
A comparison of the samples color for a series of standards was used to determine the concentration of ammonia. Equal volumes of the sample and standards were transferred to a set of tubes with flat bottoms. The tubes were placed in a rack equipped at the bottom with a reflecting surface, allowing light to pass through the solution. The colors of the samples and standards were compared by looking down through the solutions. A modified form of this method is used for the analysis of ammonia in water and wastewater.
Hanna Product Overview
The Checker HC series is the world’s first handheld colorimeter and was brought to market in 2010. This single parameter, palm-sized colorimeter was the first of its kind. Before the Checker HC, colorimeters were either an expensive ($200-300) portable photometer or an inexpensive chemical test kit. The chemical test kits offer advantage of being inexpensive but do not provide the high degree of resolution or the non-subjective nature of a photometer.
Each Checker is designed for a single parameter measurement. All of our Checkers are based on chemical methods with a specific wavelength. For example, the HI772 Marine Alkalinity dKH Checker has a LED @ 610 nm while the HI736 Marine Ultra Low Range Phosphorus Checker has LED @ 525 nm. Each method requires a specific wavelength for maximum accuracy and repeat-ability. This is why all of the Checkers are sold as individual kits, versus selling one unit that measures all parameters.
The more advanced HI83303 Aquaculture Photometer and benchtop photometer series utilizes an advanced optical system with multiple LEDs to select the correct wavelength for each preprogramed method. Photometers have a more advanced optical system compared to Checkers, but utilize the same principles of colorimetry.

The internal reference system (reference detector) of the HI83303 photometer compensates for any drifts due to power fluctuations or ambient temperature changes, providing a stable source of light for your blank (zero) measurement and sample measurement. LED light sources offer superior performance compared to tungsten lamps. LEDs have a much higher luminous efficiency, providing more light while using less power. They also produce little heat, which could otherwise affect electronic stability. Improved optical filters ensure greater wavelength accuracy and allow a stronger signal to be received.
The end result is higher measurement stability and less wavelength error. A focusing lens collects all of the light that exits the cuvette, mitigating errors from cuvette imperfections and scratches, eliminating the need to index the cuvette. The HI83303 offers an absorbance measuring mode that allows for CAL Check standards to be used to validate the performance of the system. The absorbance mode allows the user to select one of the five wavelengths of light (420 nm, 466 nm, 525 nm, 575 nm, and 610 nm) to measure and plot their own concentration versus absorbance mode. The Aquaculture Photometer can also utilize a pH electrode, log/export testing data and measures 20 different programmed methods measuring 12 key water quality parameters.
Improved Optical System found in HI83303 Aquaculture Photometer
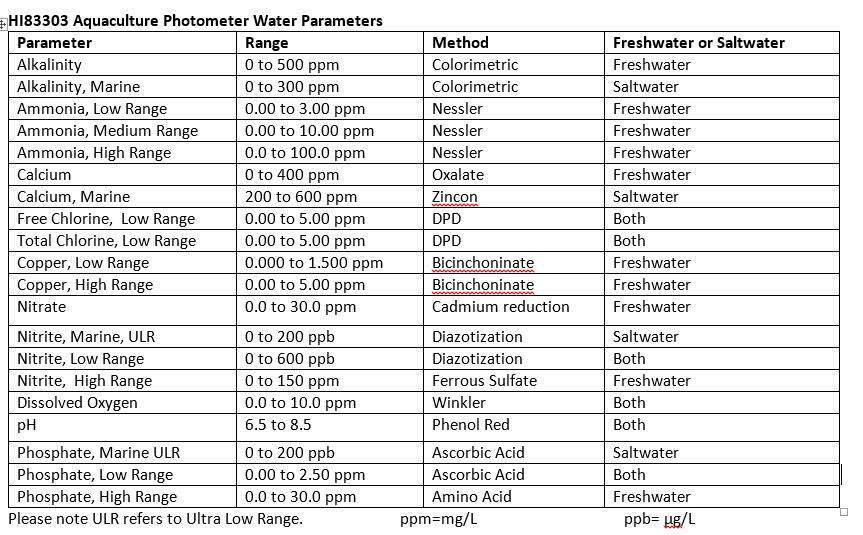
The following table outlines the various parameters and methods programmed installed onto the HI83303 Aquaculture Photometer. For a complete list of reagents please see page 82 of the product manual found here. Note the parameters labeled Marine utilize the same reagents as the Marine Line of Checkers. Products labeled freshwater have not been tested/designed to be used in saltwater, so accuracy is unknown if used in the reef tank.
Conclusion
Photometers and colorimeters provide a consistent, easy, and affordable way to get precision and accuracy in your water testing. Having a Checker allows the reef aquarist to own a lab style instrument at a hobbyist price point.
Although chemical test kits can be an effective way to measure necessary parameters in your reef tank, there is no way to quantify inaccuracies from human error or interpretation to color changes. What is blue to one person might be purple to another and thus how do we determine what the margin of error is? With our Checkers, or photometers in general, accuracy statements are clearly defined so the user can rely more confidently on their test. It’s important to remember that Checkers HC, colorimeters and photometers do require certain troubleshooting steps not found in regular chemical test kits.
For an overview of steps to follow please visit our blog on 8 Checker Best Practices. Thank you for reading and please contact 401-765-7500 or email [email protected] for questions.
By: Kevin Costa, Hanna Instruments
Intro
Maintaining water quality and critical parameters is the single most important aspect of having a successful aquarium. Knowing your water's parameters is usually the first troubleshooting step to any health issue arising in aquatic life and is vital to maintaining sensitive fish, coral, or other ornamentals. As aquarium technologies develop, so do the tools we use to test our water. Hobbyists are no longer constrained to simple chemical test kits, but now have a multitude of options ranging in scientific complexity to analyze their reef tank’s water chemistry.
One tool used widely by reefers is our Hanna Checker. The Hanna Checker® HC Series has been a valuable resource for reefkeepers to accurately test their aquaria. The HC stands for handheld colorimeter, because they utilize the principles of colorimetry for testing. The Checker HCs bridge the gap between simple chemical test kits and professional instrumentation. By using a fixed light source, proven chemical method, and dedication to a single parameter, we have provided an affordable, accurate, and easy to use testing system that rivals other instruments that cost thousands of dollars. But have you ever wondered what actually is going on inside your Checker? Colorimeters are commonly used in a variety of applications and measure absorbance from specific wavelengths of light in conjunction with certain reagents. Learn more below to understand how your Checker works, the principles of colorimetry, and other photometric instruments.
Light and Color
Before entering into colorimetry, it’s important to understand the relationship between light and color. In simple terms, colors are dependent on light. When white light shines on an object, it may be reflected, absorbed, and/or transmitted. Glass transmits most of the light that comes into contact with it, thus it appears colorless. Snow reflects all of the light and appears white. A black cloth absorbs all light, so it appears black. A red piece of paper reflects red light better than it reflects other colors.
Objects appear colored because their chemical structure absorbs certain wavelengths of light and reflects others. The wavelengths of visible light that are reflected are what we see.
When discussing light, we are usually referring to white (visible) light. When white light passes through a prism (a triangular transparent object) the colors that make up white light disperse into seven bands of color. These bands of color are called a spectrum. Seven primary colors make up white light: red, orange, yellow, green, blue, indigo, and violet.
Suppose we shine a beam of white light at a substance that absorbs blue light. Since the blue component of the white light gets absorbed by the substance, the light that is transmitted is mostly yellow, the complementary color of blue. This yellow light reaches our eyes, and we “see” the substance as a yellow colored substance.
The color variation of a system that undergoes a change in concentration of some component is the basis of colorimetric analysis.
Colorimetry
Colorimetry is simply the measurement of color; it is the determination of the concentration of a substance by measurement of the relative absorption of light with respect to a known concentration of the substance. In visual colorimetry, natural or artificial white light is generally used as a light source and determinations are usually made with a instrument called a colorimeter, or color comparator. When the eye is replaced by a photoelectric cell, the instrument is termed a photoelectric colorimeter.
A colorimetric analysis is based on the principle that many substances react with each other and form a color with an intensity that correlates to the concentration of the substance to be measured. When a substance is exposed to a beam of light of intensity (I₀) a portion of the radiation is absorbed by the substance’s molecules and a radiation of intensity (I) is emitted. This difference in intensity is used for the colorimetric determination.
The quantity of radiation absorbed is given by the Beer-Lambert Law:
Absorbance is also given by: A= ελ • C • l where:
A is a dimensionless number
ελ the proportionality constant, is called the molar extinction coefficient or molar absorptivity; it’s a constant for a given substance, provided the temperature and wavelength are constant [L/(mol•cm)]
C concentration of the substance (mol/liter)
l optical distance light travels through sample (cm)
Therefore, the concentration (C) can be calculated from the absorbance of the substance determined by the emitted radiation (I), as the other factors are known.
A typical block diagram of a photometer is shown below:
Sources of light used by Hanna colorimeters:
-Tungsten lamp: an incandescent lamp having a tungsten filament
-LED light emitting diode
The optical distance is measured by the dimension of the cuvette containing the sample. The photoelectric cell collects the radiation (I) emitted by the sample and converts it into an electric current, producing a potential in the millivolt (mV) range. The microprocessor uses this potential to convert the incoming value into the desired measuring unit and display it on the LCD. In fact, the preparation of the solution to be measured occurs under known conditions, which are programmed into the meter’s microprocessor in the form of a calibration curve. This curve is used as a reference for each measurement. It’s then possible to determine unknown concentrations of a sample by using a colorimetric reaction and the mV signal separated by a sensor in relation to the emitted intensity (I) (the color of the sample). By employing the calibration curve, you can determine the concentration of the sample that corresponds to the mV value. Supposing that for one chemical substance there is a maximum absorbance at 610 nm. I represents the light intensity after passing through a reacted sample while (I₀) equals the light intensity after passing an unreacted sample. As light passes through a solution, some is absorbed while some is transmitted through a solution. The difference between I₀ and I is used to determine the concentration. It’s important to also establish a zero readings before adding reagent to the sample to compensate for any color present prior to reagent being added.
Absorbance also depends upon the concentration of the absorbing compound in the path length of the light through the cuvette. Thus differences in the path length can effect absorbance. Since cuvettes are not uniformly round, this can have an impact on path length. Indexing the cuvettes in the same position from C1 to C2 helps make sure the path length is the same and creates minimal variation in results from C1 to C2. Furthermore, we also want to keep the cuvettes free of scratches, fingerprints, smudges, or any imperfections which can impede the light, and have an impact on results. With the following graphs, you have one example of how the colorimeters are working to determine concentration:
One example of an early colorimetric analysis is Nessler’s method for ammonia, which was first proposed in 1856. Nessler found that adding an alkaline solution of HgI2 and KI to a dilute solution of ammonia produced a yellow to reddish brown colloid, with the color intensity proportional to the concentration of ammonia present.
A comparison of the samples color for a series of standards was used to determine the concentration of ammonia. Equal volumes of the sample and standards were transferred to a set of tubes with flat bottoms. The tubes were placed in a rack equipped at the bottom with a reflecting surface, allowing light to pass through the solution. The colors of the samples and standards were compared by looking down through the solutions. A modified form of this method is used for the analysis of ammonia in water and wastewater.
Hanna Product Overview
The Checker HC series is the world’s first handheld colorimeter and was brought to market in 2010. This single parameter, palm-sized colorimeter was the first of its kind. Before the Checker HC, colorimeters were either an expensive ($200-300) portable photometer or an inexpensive chemical test kit. The chemical test kits offer advantage of being inexpensive but do not provide the high degree of resolution or the non-subjective nature of a photometer.
Each Checker is designed for a single parameter measurement. All of our Checkers are based on chemical methods with a specific wavelength. For example, the HI772 Marine Alkalinity dKH Checker has a LED @ 610 nm while the HI736 Marine Ultra Low Range Phosphorus Checker has LED @ 525 nm. Each method requires a specific wavelength for maximum accuracy and repeat-ability. This is why all of the Checkers are sold as individual kits, versus selling one unit that measures all parameters.
The more advanced HI83303 Aquaculture Photometer and benchtop photometer series utilizes an advanced optical system with multiple LEDs to select the correct wavelength for each preprogramed method. Photometers have a more advanced optical system compared to Checkers, but utilize the same principles of colorimetry.
The internal reference system (reference detector) of the HI83303 photometer compensates for any drifts due to power fluctuations or ambient temperature changes, providing a stable source of light for your blank (zero) measurement and sample measurement. LED light sources offer superior performance compared to tungsten lamps. LEDs have a much higher luminous efficiency, providing more light while using less power. They also produce little heat, which could otherwise affect electronic stability. Improved optical filters ensure greater wavelength accuracy and allow a stronger signal to be received.
The end result is higher measurement stability and less wavelength error. A focusing lens collects all of the light that exits the cuvette, mitigating errors from cuvette imperfections and scratches, eliminating the need to index the cuvette. The HI83303 offers an absorbance measuring mode that allows for CAL Check standards to be used to validate the performance of the system. The absorbance mode allows the user to select one of the five wavelengths of light (420 nm, 466 nm, 525 nm, 575 nm, and 610 nm) to measure and plot their own concentration versus absorbance mode. The Aquaculture Photometer can also utilize a pH electrode, log/export testing data and measures 20 different programmed methods measuring 12 key water quality parameters.
Improved Optical System found in HI83303 Aquaculture Photometer
The following table outlines the various parameters and methods programmed installed onto the HI83303 Aquaculture Photometer. For a complete list of reagents please see page 82 of the product manual found here. Note the parameters labeled Marine utilize the same reagents as the Marine Line of Checkers. Products labeled freshwater have not been tested/designed to be used in saltwater, so accuracy is unknown if used in the reef tank.
Conclusion
Photometers and colorimeters provide a consistent, easy, and affordable way to get precision and accuracy in your water testing. Having a Checker allows the reef aquarist to own a lab style instrument at a hobbyist price point.
Although chemical test kits can be an effective way to measure necessary parameters in your reef tank, there is no way to quantify inaccuracies from human error or interpretation to color changes. What is blue to one person might be purple to another and thus how do we determine what the margin of error is? With our Checkers, or photometers in general, accuracy statements are clearly defined so the user can rely more confidently on their test. It’s important to remember that Checkers HC, colorimeters and photometers do require certain troubleshooting steps not found in regular chemical test kits.
For an overview of steps to follow please visit our blog on 8 Checker Best Practices. Thank you for reading and please contact 401-765-7500 or email [email protected] for questions.
Last edited:









