There's a common pattern in DIY reefing: (1) I buy dry powder to dose some ion/anion, usually from LoudWolf. (2) I need to dilute it to make a solution. (3) I dose some amount of the solution.
Give a man a fish
I usually search and find some rule of thumb Randy has said (see attachment) and then monkey with the proportions, cross multiply a couple things, get confused and finally get a (maybe correct) answer.
...vs Teach a man to fish
How do I calculate it just with basic info about the molecule? For example, how do I calculate that trisodium phosphate is "about 58% phosphate"? And then, given the density, calculate how much water to make the solution, the size of each dose of that solution, and the estimated effect on my tank?
For example, let's use Na3PO4:
[given basic info/molar mass/density of Na3PO4]
Tank volume = 123.45 liters
Dry Powder volume = p ml
... Diluted with w ml of distilled water
... will increase my phosphate by x ppm if I dose d ml of my solution.
In general, what's the correct way to solve for one variable given the others?
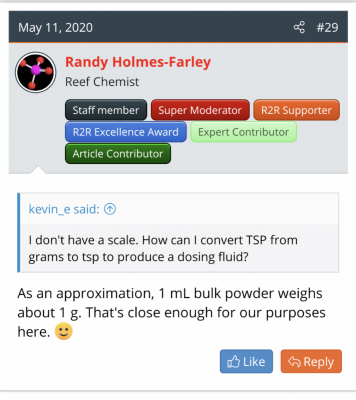
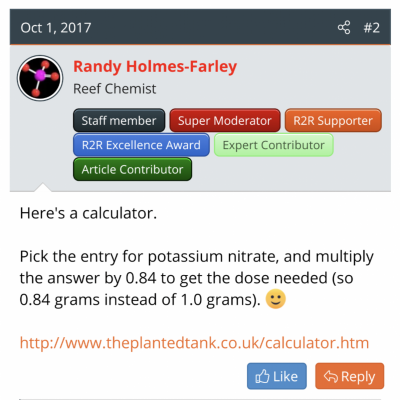
Give a man a fish
I usually search and find some rule of thumb Randy has said (see attachment) and then monkey with the proportions, cross multiply a couple things, get confused and finally get a (maybe correct) answer.
...vs Teach a man to fish
How do I calculate it just with basic info about the molecule? For example, how do I calculate that trisodium phosphate is "about 58% phosphate"? And then, given the density, calculate how much water to make the solution, the size of each dose of that solution, and the estimated effect on my tank?
For example, let's use Na3PO4:
[given basic info/molar mass/density of Na3PO4]
Tank volume = 123.45 liters
Dry Powder volume = p ml
... Diluted with w ml of distilled water
... will increase my phosphate by x ppm if I dose d ml of my solution.
In general, what's the correct way to solve for one variable given the others?