- Joined
- Dec 28, 2016
- Messages
- 22,742
- Reaction score
- 21,911
Here are the stats for the model
Abs @ 656 = NO3 + PO4 + Constant.
The interaction term was not statistically significant. R2 for the model was 0.6.
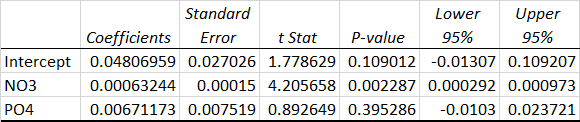
What do you think?
I dont know what intercept is. I would just take the mean and SD and do a t-test. (is that what you did)? Again - its unclear which tubes you're comparing?


















