As I was thinking on this, I started to chuckle. If you recall the movie Idiocracy, the cure to everything was supposed to be electrolytes from "Brawndo," which when put on plants obviously killed the plants. So, maybe my idea is for pouring Brawndo into my tank, with expected bad results.
However, as electrolysis seems to break H2O into oxygen (plants and animals love oxygen) and Hydrogen (pH rises with more hydrogen), it seemed somewhat intuitive that maybe that aspect of hydrolysis might be good for reef tanks, both to raise pH and to raise oxygen, both of which seem good. Granted, bubbling out would not gain anything, but maybe a "hydrolysis reactor" attachment might somehow keep the oxygen and hydrogen in solution, maybe in the ATO or something, to raise pH?
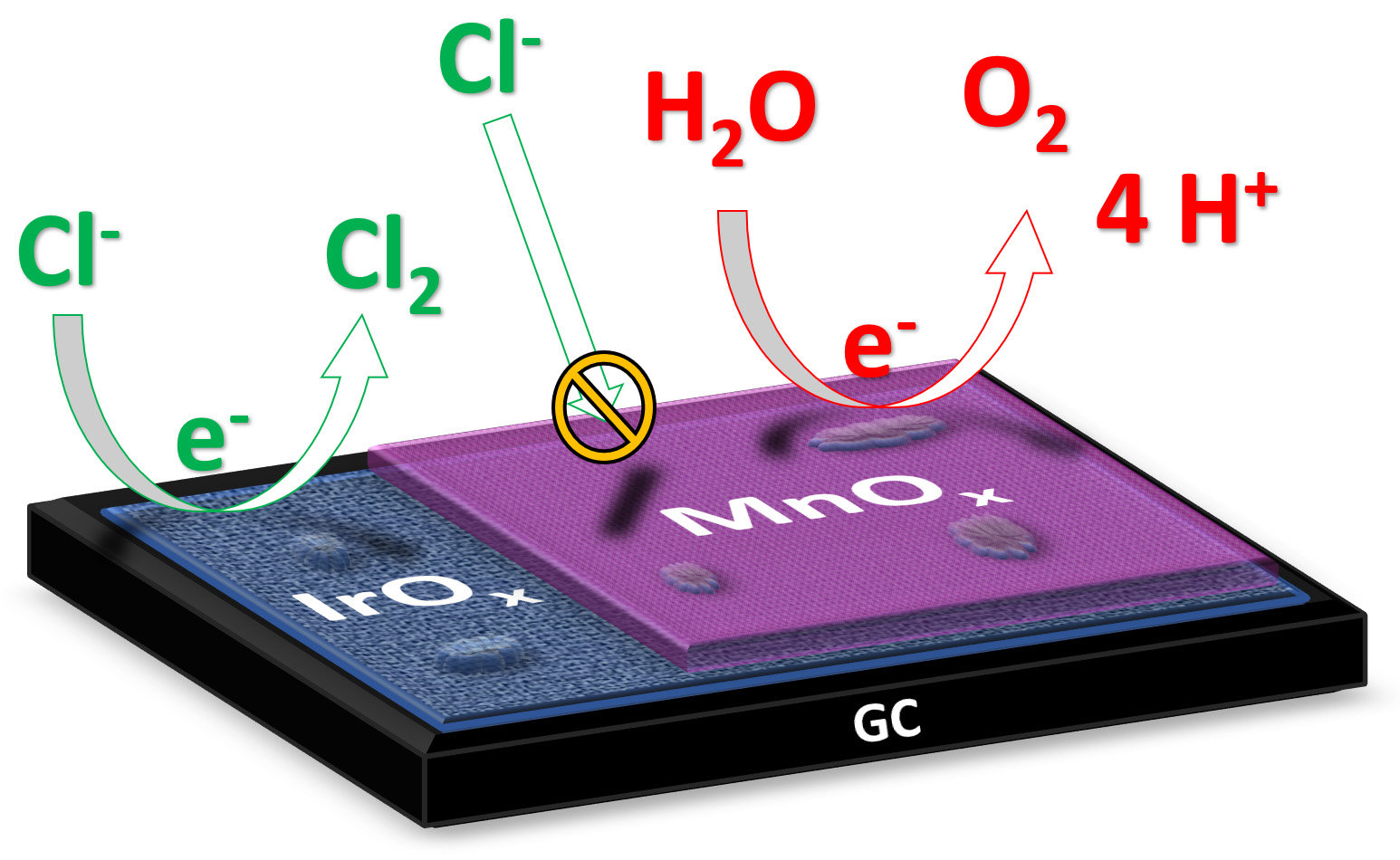
The obvious problem seems to be that NaCl when used as the medium apparently causes free chlorine also, which is great for swimming pools but not so much for reef tanks. However, that's when I thought about the ATO, where maybe the ATO water could be made hyper-base water early enough for the Na and Cl to group back together, or gas off, or something. Or, maybe some reactor device could somehow use some sort of mechanism or something to clean out the bad stuff before the hydrogen and oxygen went into the tank (like a magnet to catch the sodium, gas off the chlorine).
On that note, why not just simply dose hydrogen gas (other than the obvious Hindenburg tank risk)?
There has to be an obvious reason why all of this is nuts, but it might be fun for someone with knowledge to point out how ignorant my thinking truly is.
However, as electrolysis seems to break H2O into oxygen (plants and animals love oxygen) and Hydrogen (pH rises with more hydrogen), it seemed somewhat intuitive that maybe that aspect of hydrolysis might be good for reef tanks, both to raise pH and to raise oxygen, both of which seem good. Granted, bubbling out would not gain anything, but maybe a "hydrolysis reactor" attachment might somehow keep the oxygen and hydrogen in solution, maybe in the ATO or something, to raise pH?
The obvious problem seems to be that NaCl when used as the medium apparently causes free chlorine also, which is great for swimming pools but not so much for reef tanks. However, that's when I thought about the ATO, where maybe the ATO water could be made hyper-base water early enough for the Na and Cl to group back together, or gas off, or something. Or, maybe some reactor device could somehow use some sort of mechanism or something to clean out the bad stuff before the hydrogen and oxygen went into the tank (like a magnet to catch the sodium, gas off the chlorine).
On that note, why not just simply dose hydrogen gas (other than the obvious Hindenburg tank risk)?
There has to be an obvious reason why all of this is nuts, but it might be fun for someone with knowledge to point out how ignorant my thinking truly is.