Dear Reefers,
This is just a very quick “technical note”, and I have not included a lot of detail. If you need more information you can contact me at [email protected] or here in the forum.
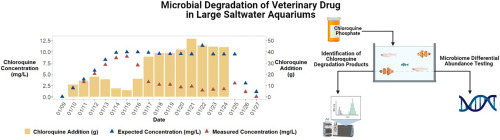
Some time ago Robert (Humblefish) contacted me about the analysis of chloroquine in seawater, and also supplied samples from his quarantine tanks (Thank you very much!).
Chloroquine is commonly used as medication against several parasitic diseases (such as Cryptocaryon or Amyloodinium) in marine fish treatment & quarantine.
It is essential to keep the concentration of medications within the therapeutic range throughout the treatment period to interrupt the life cycle of the parasites. Chloroquine concentration within the tank water might decline due to bacterial metabolism, precipitation, light induced degradation and other mechanisms. - For this reason, it is important to measure (and adjust) the concentration of active ingredient on a regular basis.
Chloroquine strongly absorbs light at around 340 nm – and it is common practice to measure this light absorbance using a photometer to evaluate the actual chloroquine concentration. In photometry all compounds present in the mixture that absorb light at the given wavelength contribute to the measurement.
At the Oceamo laboratory we have been using C18-HPLC (High Performance Liquid Chromatography) with UV detection at 343 nm to analyze Roberts “real world” samples from chloroquine-dosed tanks. (For people not familiar with the technique: The water sample is injected into a column, and within this column different chemical compounds are separated and individually detected.) This way it is possible (in contrast to photometry) to evaluate if there are different compounds present within the sample, that absorb light at a given wavelength.
Exemplary chromatogram/results:
Three chemical species are present in typical samples consisting of chloroquine, as well as two different metabolites. The main metabolite (“Metabolite 1”) was identified by LC/MS (liquid chromatography/mass spectrometry) as deaminated & oxidized species that is very likely to lack biological activity.
This exemplary HPLC chromatogram shows the sample of water was taken from a non-illuminated quarantine tank dosed with chloroquine (20 mg/l) seven days prior to sampling. As you can see in the chromatogram only a fraction is actual chloroquine, while metabolite 1 is the major chemical species present in the sample.
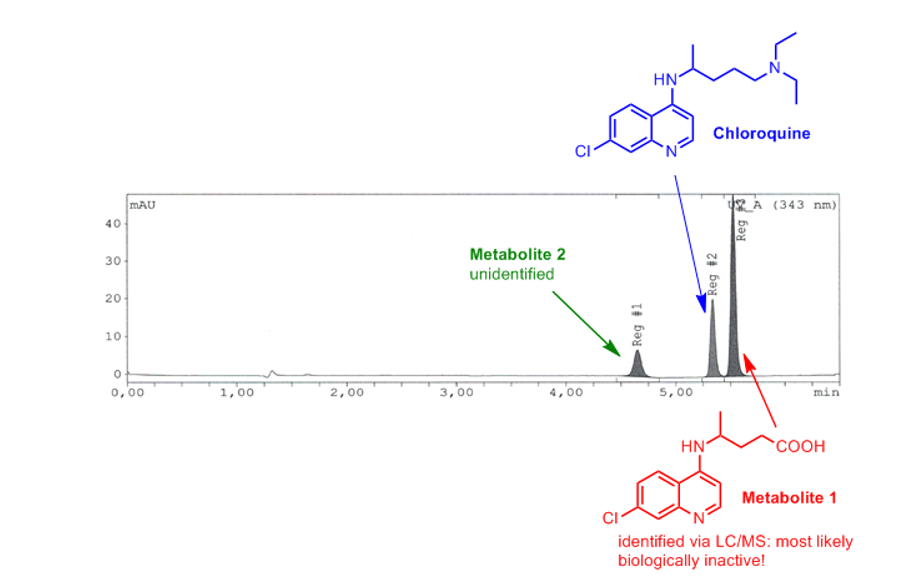
This finding clearly shows that photometry is not suitable to evaluate the chloroquine concentration in treated tanks. Metabolites that are rapidly formed (and that do likely not show any therapeutic efficiency) can not be distinguished from chloroquine using this method.
Unfortunately the number of samples was not big enough to evaluate which factors (light or bacterial activity) cause the degradation of chloroquine, and how it can be prevented. Also it is not possible determine the “half-life” of chloroquine in seawater, since the kinetics of degradation can vary greatly between different setups. Anyhow, its is planned to get more data and insight on this topic.
Best regards,
Christoph
This is just a very quick “technical note”, and I have not included a lot of detail. If you need more information you can contact me at [email protected] or here in the forum.
Some time ago Robert (Humblefish) contacted me about the analysis of chloroquine in seawater, and also supplied samples from his quarantine tanks (Thank you very much!).
Chloroquine is commonly used as medication against several parasitic diseases (such as Cryptocaryon or Amyloodinium) in marine fish treatment & quarantine.
It is essential to keep the concentration of medications within the therapeutic range throughout the treatment period to interrupt the life cycle of the parasites. Chloroquine concentration within the tank water might decline due to bacterial metabolism, precipitation, light induced degradation and other mechanisms. - For this reason, it is important to measure (and adjust) the concentration of active ingredient on a regular basis.
Chloroquine strongly absorbs light at around 340 nm – and it is common practice to measure this light absorbance using a photometer to evaluate the actual chloroquine concentration. In photometry all compounds present in the mixture that absorb light at the given wavelength contribute to the measurement.
At the Oceamo laboratory we have been using C18-HPLC (High Performance Liquid Chromatography) with UV detection at 343 nm to analyze Roberts “real world” samples from chloroquine-dosed tanks. (For people not familiar with the technique: The water sample is injected into a column, and within this column different chemical compounds are separated and individually detected.) This way it is possible (in contrast to photometry) to evaluate if there are different compounds present within the sample, that absorb light at a given wavelength.
Exemplary chromatogram/results:
Three chemical species are present in typical samples consisting of chloroquine, as well as two different metabolites. The main metabolite (“Metabolite 1”) was identified by LC/MS (liquid chromatography/mass spectrometry) as deaminated & oxidized species that is very likely to lack biological activity.
This exemplary HPLC chromatogram shows the sample of water was taken from a non-illuminated quarantine tank dosed with chloroquine (20 mg/l) seven days prior to sampling. As you can see in the chromatogram only a fraction is actual chloroquine, while metabolite 1 is the major chemical species present in the sample.
This finding clearly shows that photometry is not suitable to evaluate the chloroquine concentration in treated tanks. Metabolites that are rapidly formed (and that do likely not show any therapeutic efficiency) can not be distinguished from chloroquine using this method.
Unfortunately the number of samples was not big enough to evaluate which factors (light or bacterial activity) cause the degradation of chloroquine, and how it can be prevented. Also it is not possible determine the “half-life” of chloroquine in seawater, since the kinetics of degradation can vary greatly between different setups. Anyhow, its is planned to get more data and insight on this topic.
Best regards,
Christoph