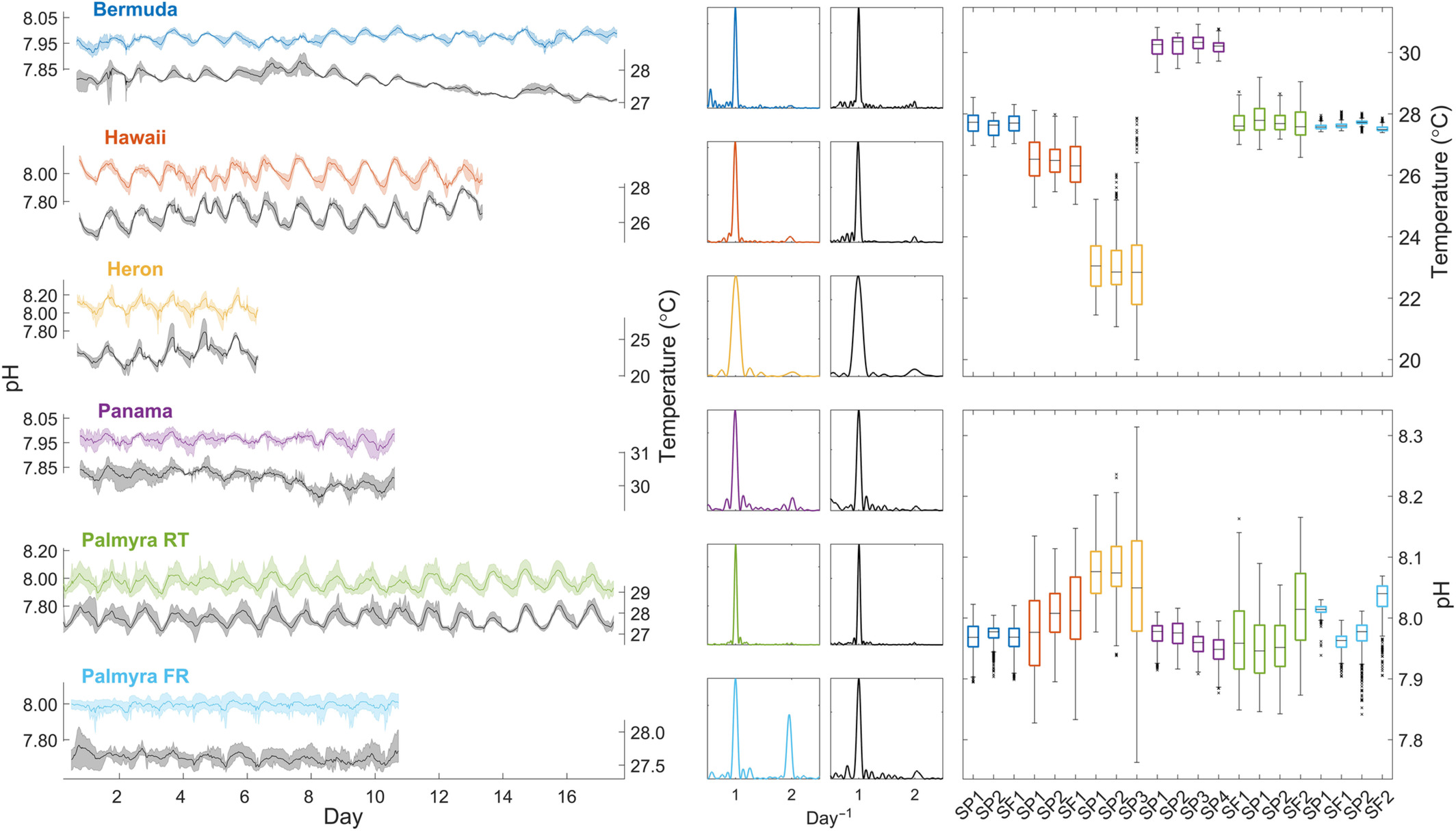
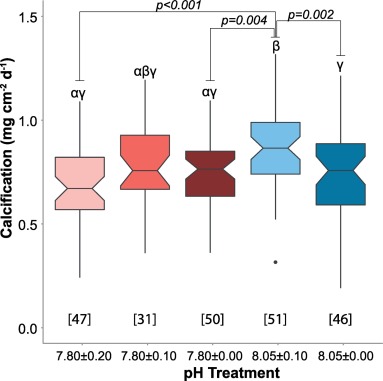
It is well-accepted that corals (SPS, in particular), grow faster at a higher pH (8.2-8.3 compared to 7.8) (assuming other parameters are kept optimal) and that stability is one of the main "keys" to successful coral growth. Can that "stability" be extended to pH? In other words, would it be beneficial to maintain pH at a constant level (8.2 or 8.3) rather than allowing the more "normal" diurnal fluctuation? …..and, what would that ideal pH be? (Let's disregard the effort/difficulty involved in keeping the pH constant).
Obviously, aquarists have had much success with growing corals over the decades with allowing "normal" diurnal fluctuation of pH, but my question is: Would a constant pH be even MORE beneficial, or would it be more likely to be harmful? I know it has been suggested that different organisms/(corals) may prefer different pH's, but if my concern is only with SPS, I'm wondering if a constant pH of 8.3 would be harmful or beneficial.
I've devised a way to maintain a constant pH of 8.3 by plumbing and controlling 3 tubes to my skimmer:
1) Room air (High CO2)
2) Outside air (Low CO2)
3) Outside air with CO2 scrubber (Very Low CO2).
At the present time, since I'm trapped at home because of Covid and have time on my hands, I'm controlling the 3 inputs with manual valves ("by hand") throughout the day but have 2 motorized ball valves arriving soon. At the moment, I'm maintaining the pH most of the time right at 8.3 but with occasional fluctuations between 8.27 and 8.34. Once I get the motorized ball valves and with the help of my GHL Controller, I expect to be able to maintain the pH 8.3 with a 0.05 hysteresis, so, 8.28-8.33. (I may discuss the details of this set-up at a later date on a different Forum since such a discussion would be more appropriate for a DIY Forum rather than Randy's Chemistry Forum).
So, I would welcome any suggestions or responses to my questions above (in bold print). It might be helpful to state whether your replies/opinions are based on scientific literature or anecdotal, when appropriate.
Thanks for helping!
Obviously, aquarists have had much success with growing corals over the decades with allowing "normal" diurnal fluctuation of pH, but my question is: Would a constant pH be even MORE beneficial, or would it be more likely to be harmful? I know it has been suggested that different organisms/(corals) may prefer different pH's, but if my concern is only with SPS, I'm wondering if a constant pH of 8.3 would be harmful or beneficial.
I've devised a way to maintain a constant pH of 8.3 by plumbing and controlling 3 tubes to my skimmer:
1) Room air (High CO2)
2) Outside air (Low CO2)
3) Outside air with CO2 scrubber (Very Low CO2).
At the present time, since I'm trapped at home because of Covid and have time on my hands, I'm controlling the 3 inputs with manual valves ("by hand") throughout the day but have 2 motorized ball valves arriving soon. At the moment, I'm maintaining the pH most of the time right at 8.3 but with occasional fluctuations between 8.27 and 8.34. Once I get the motorized ball valves and with the help of my GHL Controller, I expect to be able to maintain the pH 8.3 with a 0.05 hysteresis, so, 8.28-8.33. (I may discuss the details of this set-up at a later date on a different Forum since such a discussion would be more appropriate for a DIY Forum rather than Randy's Chemistry Forum).
So, I would welcome any suggestions or responses to my questions above (in bold print). It might be helpful to state whether your replies/opinions are based on scientific literature or anecdotal, when appropriate.
Thanks for helping!