- Joined
- Dec 28, 2016
- Messages
- 22,852
- Reaction score
- 21,984
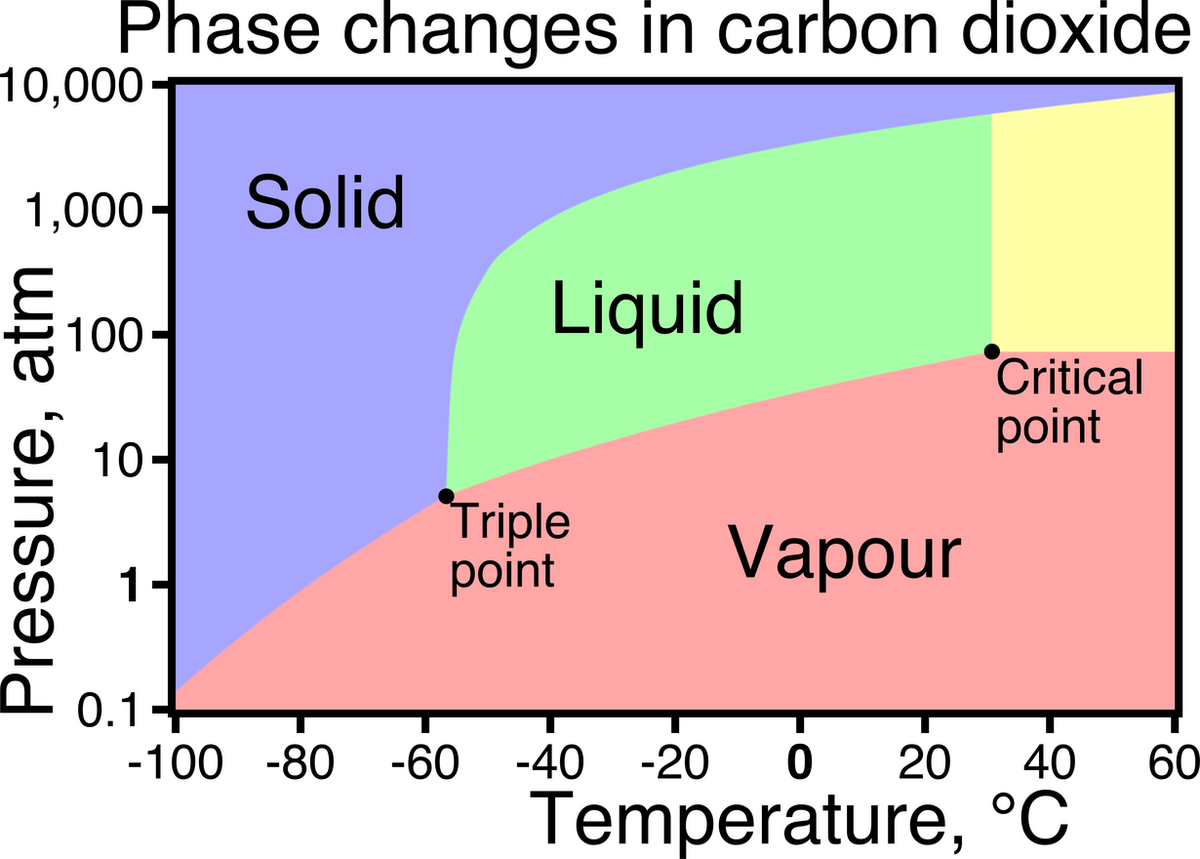
Do you mean a compound or an element. IMHO - 'liquid ammonia' unless under high pressure - is dissolved in water - it's not actually 'liquid ammonia'. Same with CO2. And they are not elements. Same with H2O. And I don't know of anyone thats more concerned today vs 10 years ago about H2O,, CO2 or Ammonia?Is this a single compound? I wanted to say co2 but clearly carbonic acid isn't co2.
If it's a single compound, then this is a tough one. First thought was ammonia or co2, but my guess is a different compound or aqueous solution isn't the answer here.