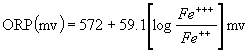Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,391
- Reaction score
- 63,732
Reef Chemistry Question of the Day 299
You are making a batch of new salt water for a water change, and realize that you overshot your salinity target. You measure the parameters to be:
salinity 36 ppt
pH 8.0
Calcium 455 ppm
Alkalinity 9 dKH
Magnesium 1380 ppm
ORP 255 mV
You then add some additional 0 ppm TDS RO/DI water straight from the RO/DI outlet.
Which of the following parameter changes in the new salt water is impossible as a consequence of that addition?
Pick all that are impossible.
1. Salinity rises
2. pH rises
3. Calcium falls
4. Alkalinity falls
5. Magnesium falls
5. ORP falls
Good luck and Happy Reefing!
Previous Reef Chemistry Question of the day:

 www.reef2reef.com
www.reef2reef.com
You are making a batch of new salt water for a water change, and realize that you overshot your salinity target. You measure the parameters to be:
salinity 36 ppt
pH 8.0
Calcium 455 ppm
Alkalinity 9 dKH
Magnesium 1380 ppm
ORP 255 mV
You then add some additional 0 ppm TDS RO/DI water straight from the RO/DI outlet.
Which of the following parameter changes in the new salt water is impossible as a consequence of that addition?
Pick all that are impossible.
1. Salinity rises
2. pH rises
3. Calcium falls
4. Alkalinity falls
5. Magnesium falls
5. ORP falls
Good luck and Happy Reefing!
Previous Reef Chemistry Question of the day:

Reef Chemistry Question of the Day #298: Where are those trace elements coming from?
Reef Chemistry Question of the Day 298 We all know that organisms from bacteria to whales require many different trace elements for the biochemical processes of life. One of the things we don't always have a good handle on in a reef tank is where they are coming from and where they are going...
 www.reef2reef.com
www.reef2reef.com