- Joined
- Nov 26, 2017
- Messages
- 8
- Reaction score
- 11
(This thread has been requested and approved by the sponsor Premium aquatics)
Do You Track Ammonia?
Hi everyone,
For as long as I can remember in this hobby it has always been perceived that once you have a mature tank Ammonia is or should never be an issue, we all instead focus on Nitrate and PO4 . However this should not be the case.
Ammonia is the first stage of the nitrogen cycle, Ammonia - Nitrite – Nitrate – Nitrogen Gas so by simple deduction if your Nitrate is rising or has gone up the ammonia level in the tank at some point would have risen also, so any tool that allows you to keep track of even the smallest ammonia levels on a daily bases must be an advantage? Having the ability to react to the slightest changes before you end up with a bigger issue along the way. And this is where the Seneye Monitors come into play as they monitor both Ammonia (the harmful component) and Ammonium (This is safe providing pH levels do not fluctuate, however go outside that safe zone and ammonium turns into Ammonia).
What is ammonia NH3 & NH4?
Ammonia from the gills of fish, their urine, and rotting food or decaying plant matter are contributors of ammonia in an aquarium.
It exists in two forms in the aquarium and the first step is to understand the difference between ammonium NH4 and free ammonia NH3.
NH3 (ammonia) is a gas and sometimes called toxic or free ammonia. This is the type of ammonia is the dangerous part.
NH4 (ammonium) is a nontoxic salt it is the ionised form of ammonia.
NH3 and NH4 together are often referred to as total ammonia nitrogen (TAN).
Under normal conditions NH3 (ammonia) and NH4 (ammonium) will both be present in aquarium water. The two exist at an equilibrium point that is governed largely by pH and temperature. However; salinity and ionic strength of the water also have influence on this equilibrium point.
The chart below shows how the ratio between NH3 and NH4 is affected by pH in a controlled sample. As the pH increases, the ionised NH4 is liberated into gaseous NH3. As the pH increases there reaches a point where NH4 cannot exist and all ammonium is presented as NH3 ammonia; this is beyond the pH of normal aquarium life.
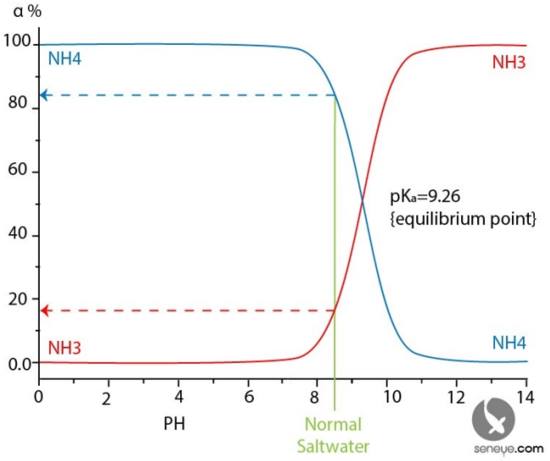
The green line on the chart below indicates the pH where a marine aquarium normally fall and shows that roughly 15% to 20% of the TAN is NH3 and the rest (80% to 85%) will be NH4. Therefore, when any ammonia (TAN) is present in a normal aquarium, the majority of it will be NH4.
Tradition test kits and photometers usually measure TAN or NH3-N and as such misinterpretation what is being measured can occur
Is an Ammonia or NH3 test kit reporting the same NH3 value as seneye?
In short, No:
Why?
Many tests use the “indophenol method” for measuring the ammonia by drastically increasing the pH of the sample. The movement in pH means that all of the ammonium (NH4) in the sample will be liberated to the NH3 form. The NH3 then reacts with the chemistry in the test to create the colour change that is measurable by the photometer or eye. The value that is outputted is either TAN or NH3-N.
This method of testing means that the NH3 level that is being measured is true of the sample after the pH increase. However, the value tells you nothing of the actual NH3 in the aquarium because the pH of the sample is not the same as the aquarium water
Often these tests allow you to use multiplication values to find NH3 or NH4 from the NH3-N value reported. This is misleading as the multipliers only allow you to put a numeric value on if all of the TAN was NH3 or NH4, not the ratio between them that is actually present in the aquarium water.
Can we do a comparison between the output of the seneye and these tests?
Yes, but caution should be taken. As a guide you should be able to take the TAN value and use a free ammonia calculator which takes into account pH, temperature, salinity and the TAN value. These calculations are based on exact chemisty and as such any minor error in the measurements of the input data will have a drastic effect on the calculated NH3 value.
This is why seneye measures NH3 directly.
There are also potential added advantages for the reef keeper struggling with Nitrate as there is evidence that at higher levels, Ammonium rises and falls as Nitrate does to, so you also have a snap shot of the complete biological cycle.
So next time test your tanks Nitrate levels have a thought as to where that Nitrate came from and would it not be ideal to be able to daily track both NH3 and NH4 independently allowing you to react with water changes even if you see 1 point rise?
To find out more about Seneye or to buy contact Premium Aquatics or click on their link to the products here
https://premiumaquatics.com/search?search=seneye
Do You Track Ammonia?
Hi everyone,
For as long as I can remember in this hobby it has always been perceived that once you have a mature tank Ammonia is or should never be an issue, we all instead focus on Nitrate and PO4 . However this should not be the case.
Ammonia is the first stage of the nitrogen cycle, Ammonia - Nitrite – Nitrate – Nitrogen Gas so by simple deduction if your Nitrate is rising or has gone up the ammonia level in the tank at some point would have risen also, so any tool that allows you to keep track of even the smallest ammonia levels on a daily bases must be an advantage? Having the ability to react to the slightest changes before you end up with a bigger issue along the way. And this is where the Seneye Monitors come into play as they monitor both Ammonia (the harmful component) and Ammonium (This is safe providing pH levels do not fluctuate, however go outside that safe zone and ammonium turns into Ammonia).
What is ammonia NH3 & NH4?
Ammonia from the gills of fish, their urine, and rotting food or decaying plant matter are contributors of ammonia in an aquarium.
It exists in two forms in the aquarium and the first step is to understand the difference between ammonium NH4 and free ammonia NH3.
NH3 (ammonia) is a gas and sometimes called toxic or free ammonia. This is the type of ammonia is the dangerous part.
NH4 (ammonium) is a nontoxic salt it is the ionised form of ammonia.
NH3 and NH4 together are often referred to as total ammonia nitrogen (TAN).
Under normal conditions NH3 (ammonia) and NH4 (ammonium) will both be present in aquarium water. The two exist at an equilibrium point that is governed largely by pH and temperature. However; salinity and ionic strength of the water also have influence on this equilibrium point.
The chart below shows how the ratio between NH3 and NH4 is affected by pH in a controlled sample. As the pH increases, the ionised NH4 is liberated into gaseous NH3. As the pH increases there reaches a point where NH4 cannot exist and all ammonium is presented as NH3 ammonia; this is beyond the pH of normal aquarium life.

The green line on the chart below indicates the pH where a marine aquarium normally fall and shows that roughly 15% to 20% of the TAN is NH3 and the rest (80% to 85%) will be NH4. Therefore, when any ammonia (TAN) is present in a normal aquarium, the majority of it will be NH4.
Tradition test kits and photometers usually measure TAN or NH3-N and as such misinterpretation what is being measured can occur
Is an Ammonia or NH3 test kit reporting the same NH3 value as seneye?
In short, No:
Why?
Many tests use the “indophenol method” for measuring the ammonia by drastically increasing the pH of the sample. The movement in pH means that all of the ammonium (NH4) in the sample will be liberated to the NH3 form. The NH3 then reacts with the chemistry in the test to create the colour change that is measurable by the photometer or eye. The value that is outputted is either TAN or NH3-N.
This method of testing means that the NH3 level that is being measured is true of the sample after the pH increase. However, the value tells you nothing of the actual NH3 in the aquarium because the pH of the sample is not the same as the aquarium water
Often these tests allow you to use multiplication values to find NH3 or NH4 from the NH3-N value reported. This is misleading as the multipliers only allow you to put a numeric value on if all of the TAN was NH3 or NH4, not the ratio between them that is actually present in the aquarium water.
Can we do a comparison between the output of the seneye and these tests?
Yes, but caution should be taken. As a guide you should be able to take the TAN value and use a free ammonia calculator which takes into account pH, temperature, salinity and the TAN value. These calculations are based on exact chemisty and as such any minor error in the measurements of the input data will have a drastic effect on the calculated NH3 value.
This is why seneye measures NH3 directly.
There are also potential added advantages for the reef keeper struggling with Nitrate as there is evidence that at higher levels, Ammonium rises and falls as Nitrate does to, so you also have a snap shot of the complete biological cycle.
So next time test your tanks Nitrate levels have a thought as to where that Nitrate came from and would it not be ideal to be able to daily track both NH3 and NH4 independently allowing you to react with water changes even if you see 1 point rise?
To find out more about Seneye or to buy contact Premium Aquatics or click on their link to the products here
https://premiumaquatics.com/search?search=seneye








