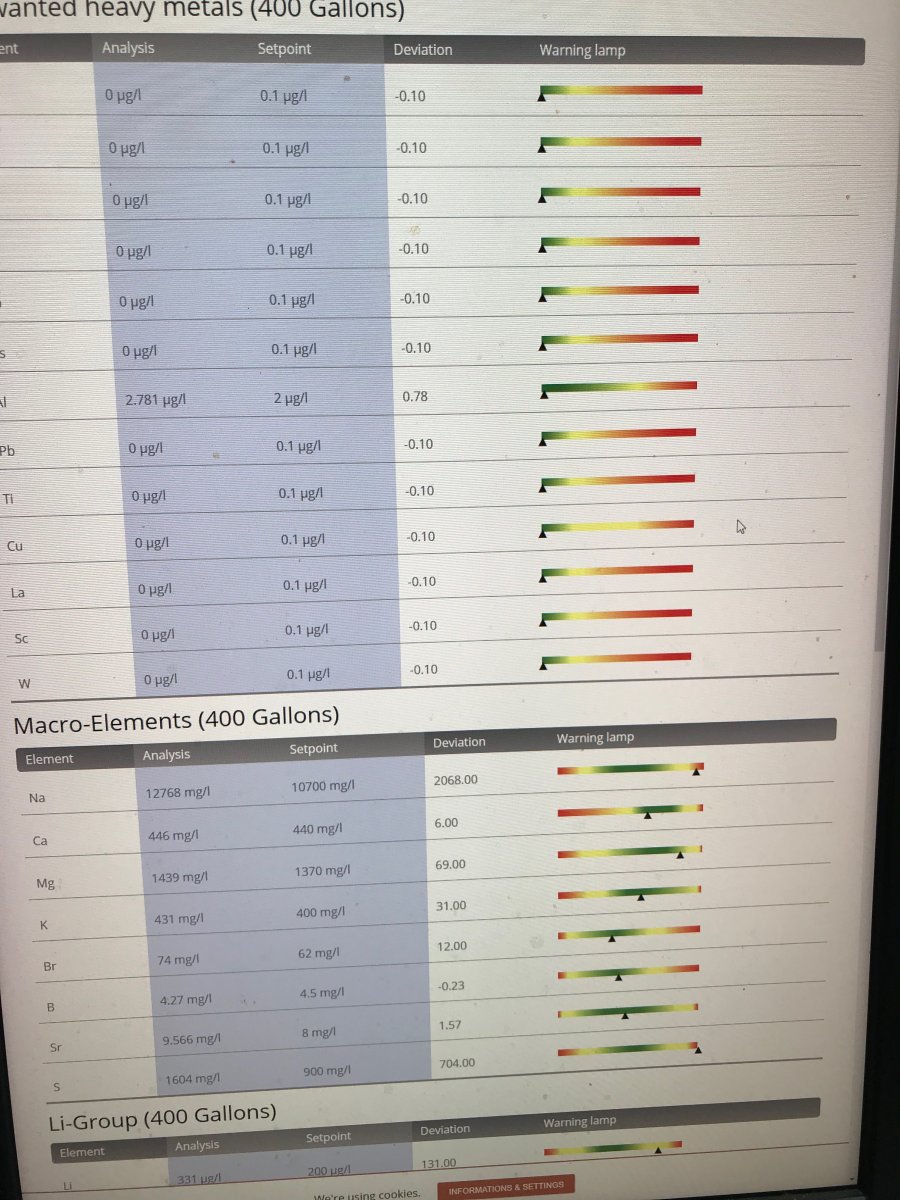
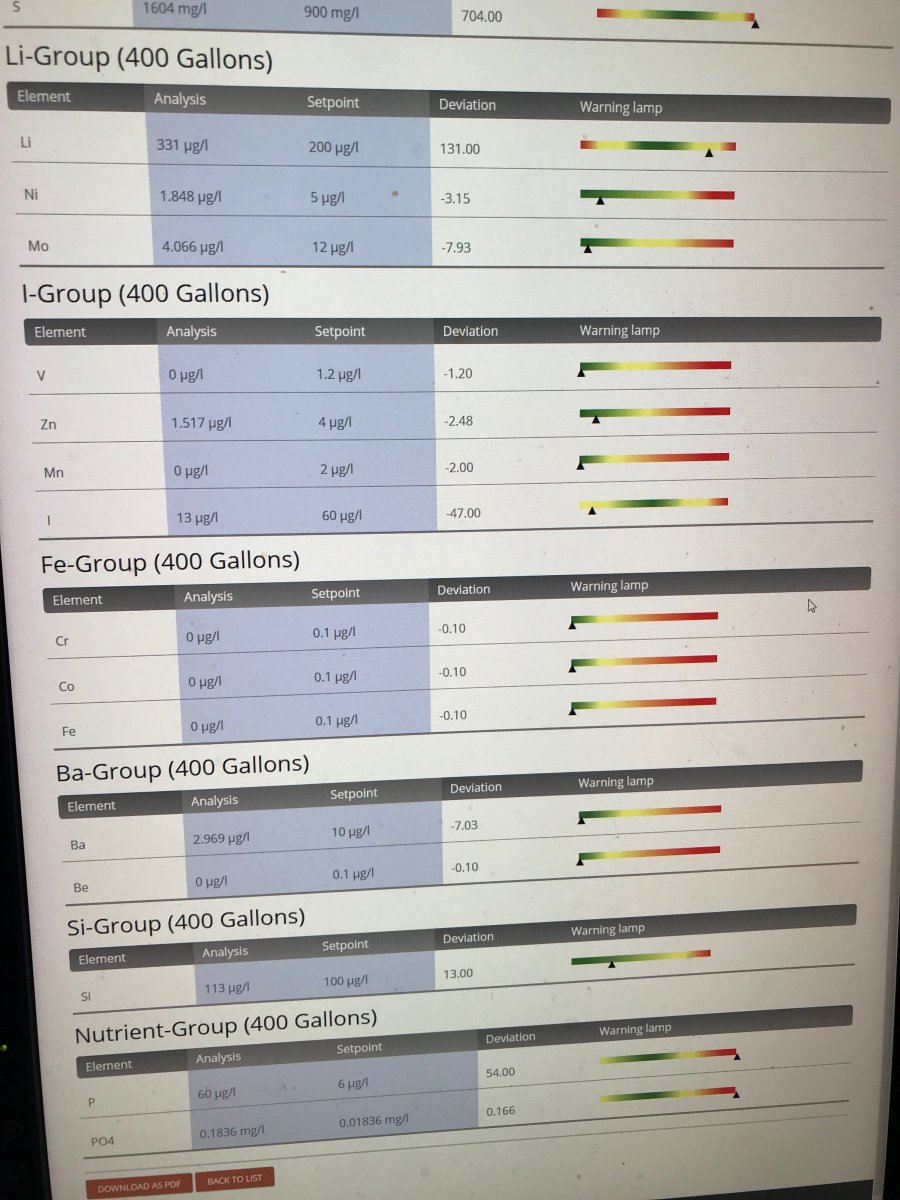
Did my annual ICP-OES water test last week. PO4 is high …. though it's always been high despite my use of GFO. Probably 125 fish is a tad too many  . Slightly lower than both 2019 or 2018, though likely within the measurement error. I don't have algae problems and the SPS grow well so I have stopped worrying about it. Li is typically high, though that seems to be a function of the IO salt mix. Likewise for Na. I am seeing high sulfur though. Not necessarily surprising since i started using a sulfur denitrator since the last test. My question is whether 1,604 mg/l (versus 900 mg/l) is something to be concerned about? I see no observational signs that the corals are stressed. May need to 'goose' the iodine dosing though.
. Slightly lower than both 2019 or 2018, though likely within the measurement error. I don't have algae problems and the SPS grow well so I have stopped worrying about it. Li is typically high, though that seems to be a function of the IO salt mix. Likewise for Na. I am seeing high sulfur though. Not necessarily surprising since i started using a sulfur denitrator since the last test. My question is whether 1,604 mg/l (versus 900 mg/l) is something to be concerned about? I see no observational signs that the corals are stressed. May need to 'goose' the iodine dosing though.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
High Sulfur
- Thread starter ca1ore
- Start date
- Tagged users None
I dont think elemental sulphur is supposed to do that, is it? Id suspect particles in the sample. Id love to see what a centrifuged sample compared to a non centrifuged sample would be.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,070
- Reaction score
- 64,516
I do not know at what level sulfate becomes undesirable. This is the highest I've seen.
The question is if the presence of sulfur is a problem. It is almost double the natural content representing sulfate. Sulfate is not considered to be a problem but does the measurement represent sulfate?
This represents +- 0.6 grams x system content in liters of sulfur consumed. In practice +- 50 % of the nitrate removed in an SD may be by using HS which closes the sulfate cycle.
+- 2.5mg sulfur is used for exporting +- 4mg nitrate, this represents +- 280ppm nitrate exported using elemental sulfur in a year's time. Total nitrate removal may be estimated to be +- 400-500 ppm,
Normally a lot of sulfates are removed changing the calcium carbonate media, it is advised to replace the calcium carbonate media at least every 3- 6 months if using a BADES application.
In practice, the sulfur consumption in the reactor does never correspond with the presence of sulfates, there should be a lot less sulfate overproduction as S which was consumed in the reactor, but certainly not more.
If the sulfur consumption is less as the S increase measured for certain something else is responsible.
The salt mix used? The water used? The measurement?
What form of S is measured? Can the result be influenced by the presence of other sulfur compounds? Most S normally is in sulfate. Using a denitrator a lot of HS may be produced by sulfate reduction but total S stays the same.
A sulfur denitrator produces sulfate.
How much elemental sulfur is consumed since the last measurement? How many water changes?
How many sulfur and calcium carbonate is used in the reactor?
How lowering sulfates? Using a low sulfate salt mix for water changes. Renewing the calcium media regularly.
Using a BADES application, using a low sulfate salt mix for normal water changes is enough for compensating the normal sulfate production in a mixed reef.
This represents +- 0.6 grams x system content in liters of sulfur consumed. In practice +- 50 % of the nitrate removed in an SD may be by using HS which closes the sulfate cycle.
+- 2.5mg sulfur is used for exporting +- 4mg nitrate, this represents +- 280ppm nitrate exported using elemental sulfur in a year's time. Total nitrate removal may be estimated to be +- 400-500 ppm,
Normally a lot of sulfates are removed changing the calcium carbonate media, it is advised to replace the calcium carbonate media at least every 3- 6 months if using a BADES application.
In practice, the sulfur consumption in the reactor does never correspond with the presence of sulfates, there should be a lot less sulfate overproduction as S which was consumed in the reactor, but certainly not more.
If the sulfur consumption is less as the S increase measured for certain something else is responsible.
The salt mix used? The water used? The measurement?
What form of S is measured? Can the result be influenced by the presence of other sulfur compounds? Most S normally is in sulfate. Using a denitrator a lot of HS may be produced by sulfate reduction but total S stays the same.
A sulfur denitrator produces sulfate.
How much elemental sulfur is consumed since the last measurement? How many water changes?
How many sulfur and calcium carbonate is used in the reactor?
How lowering sulfates? Using a low sulfate salt mix for water changes. Renewing the calcium media regularly.
Using a BADES application, using a low sulfate salt mix for normal water changes is enough for compensating the normal sulfate production in a mixed reef.
Last edited:
Oh my well i found this:
So, for every 6 moles of NO3- you get 5 moles of sulphate produced. In mass units, 1 ppm of nitrate gives 1.94 ppm of sulphate.
How much nitrate should one think will get reduced this way? I'm not sure. How much accumulates in a reef tank over time? 1 ppm per day? That would put the nitrate up 30 ppm per month.
At 1 ppm nitrate reduction per day, the sulphate will increase by about 2 ppm per day.
Normal seawater contains 2,710 ppm of sulphate.
So to get a boost of 10% in the normal sulphate concentration will take 136 days with no water changes at all.
How much change in sulphate is too much? I don't know, but let's use the variation between salt mixes as a guide to what is "OK".
In Craig's study, the sulphate ranged from 1440 ppm (Coralife; about 53% of seawater) to 3550 ppm (Seachem; about 31% more than seawater). Only 2 of the 8 salt mixes were within +/-10% of seawater. Most were substantially low. Instant Ocean was about 18% low, and could use a boost.
So, for every 6 moles of NO3- you get 5 moles of sulphate produced. In mass units, 1 ppm of nitrate gives 1.94 ppm of sulphate.
How much nitrate should one think will get reduced this way? I'm not sure. How much accumulates in a reef tank over time? 1 ppm per day? That would put the nitrate up 30 ppm per month.
At 1 ppm nitrate reduction per day, the sulphate will increase by about 2 ppm per day.
Normal seawater contains 2,710 ppm of sulphate.
So to get a boost of 10% in the normal sulphate concentration will take 136 days with no water changes at all.
How much change in sulphate is too much? I don't know, but let's use the variation between salt mixes as a guide to what is "OK".
In Craig's study, the sulphate ranged from 1440 ppm (Coralife; about 53% of seawater) to 3550 ppm (Seachem; about 31% more than seawater). Only 2 of the 8 salt mixes were within +/-10% of seawater. Most were substantially low. Instant Ocean was about 18% low, and could use a boost.
About the side effects of BADES and sulfate.
The sulfur consumption can only be used to determine how much nitrogen may have been exported but is not a guide line for the total nitrogen export as so many other processes may be involved, starting with the heterotrophic part.
Nitrate reduction due to the use of HS wich produces sulfate or and elemental sulfur. The reduction of nitrate using HS does produce a lot less sulfate for each mg nitrogen exported as when using S. 0.625 mol instead of 1.1 mol for each mol NO3-N exported. ( ref: CMF De Haes 2017)
If tap water is used to top off evaporation a lot of sulfates may enter. My tap water contains +40ppm sulfate.
If using a BADES application, it is advised to mix the sulfur with calcium carbonate media, at least 1/1. Till now, after +25 years of practical use in marine aquaria, I have no knowledge of problems caused by sulfate production due to using BADES in combination with calcium carbonate.
I do advise the use of a low sulfate salt mix for water changes and to have attention for sulfate in the top off water. https://reefhub.pl/test-of-marine-salts/
It would be nice to know if all S measured only represents sulfate or does include other sulfur compounds.
Reading the test results, my first concern would be the iron content.
The sulfur consumption can only be used to determine how much nitrogen may have been exported but is not a guide line for the total nitrogen export as so many other processes may be involved, starting with the heterotrophic part.
Nitrate reduction due to the use of HS wich produces sulfate or and elemental sulfur. The reduction of nitrate using HS does produce a lot less sulfate for each mg nitrogen exported as when using S. 0.625 mol instead of 1.1 mol for each mol NO3-N exported. ( ref: CMF De Haes 2017)
If tap water is used to top off evaporation a lot of sulfates may enter. My tap water contains +40ppm sulfate.
If using a BADES application, it is advised to mix the sulfur with calcium carbonate media, at least 1/1. Till now, after +25 years of practical use in marine aquaria, I have no knowledge of problems caused by sulfate production due to using BADES in combination with calcium carbonate.
I do advise the use of a low sulfate salt mix for water changes and to have attention for sulfate in the top off water. https://reefhub.pl/test-of-marine-salts/
It would be nice to know if all S measured only represents sulfate or does include other sulfur compounds.
Reading the test results, my first concern would be the iron content.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,070
- Reaction score
- 64,516
There certainly are other sulfur compounds, most of which are organic compounds, but they will be insignificant in concentration relative to sulfate. They would never be a reason thst total sulfur is significantly elevated.
There certainly are other sulfur compounds, most of which are organic compounds, but they will be insignificant in concentration relative to sulfate. They would never be a reason thst total sulfur is significantly elevated.
To answer the question if the S content contains a problem one should know what is measured exactly. That little part may not be "insignificant" for the total toxicity of the S level.
To connect the S content to the use of the SD other S sources must be excluded.
The situation may become an interesting study object as it shows what we do not see often if the S content has accumulated and increased +- 700ppm in a year's time.
It seems to be a yearly test. We only see the result of the latest test, it would be interesting to see the results from last year, results taken just before using the SD.
The situation may become an interesting study object as it shows what we do not see often if the S content has accumulated and increased +- 700ppm in a year's time.
It seems to be a yearly test. We only see the result of the latest test, it would be interesting to see the results from last year, results taken just before using the SD.
During an ICP-OES water test, the sample is heated, a plasma is created. What happens if the water sample contains small elemental sulfur particles? https://www.ru.nl/science/gi/facilities-activities/elemental-analysis/icp-oes/
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,070
- Reaction score
- 64,516
To answer the question if the S content contains a problem one should know what is measured exactly. That little part may not be "insignificant" for the total toxicity of the S level.
Most certainly true, But a total sulfur measurement is useless in that regard.
It would be little different than measuring total oxygen and wondering if some of that oxygen was present as phosphate.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,070
- Reaction score
- 64,516
During an ICP-OES water test, the sample is heated, a plasma is created. What happens if the water sample contains small elemental sulfur particles? https://www.ru.nl/science/gi/facilities-activities/elemental-analysis/icp-oes/
It will be detected as S.
At least one company centrifuges to remove particles before ICP.
Most certainly true, But a total sulfur measurement is useless in that regard.
It would be little different than measuring total oxygen and wondering if some of that oxygen was present as phosphate.
In that case, one measures phosphates.
In this case, the total S may contain HS. As long the presence of HS has not been excluded an answer on the question can not be given.
To determine the involvement of the BADES process one may start with the sulfur consumption.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,070
- Reaction score
- 64,516
In that case, one measures phosphates.
In this case, the total S may contain HS. As long the presence of HS has not been excluded an answer on the question can not be given.
Of course measures phosphate in other ways.
Burt H2S cannot possibly be why the sulfur is high. The tank would stink to high heaven and everything woulds be dead.
Nothing is multi ppm S, except sulfate.
Traces will be organics that contain S, such as proteins and dimethyl sulfide.
Of course measures phosphate in other ways.
Burt H2S cannot possibly be why the sulfur is high. The tank would stink to high heaven and everything woulds be dead.
Nothing is multi ppm S, except sulfate.
Traces will be organics that contain S, such as proteins and dimethyl sulfide.
Has someone claimed H2S will increase Total S?
HS does not increase Total S but the question is asked if high S may become a problem. As long one does not know what S represents one can not give a good answer.
If the tank stinks one has a much bigger problem as in a marine aquarium +90% will be HS; - 10% will be H2S.
When the water of a marine aquarium is saturated of H2S and stinks?
What is the most toxic, HS, or H2S?
If the water is saturated with H2S one will smell it.
HS may accumulate, not being part of the Total S in a water sample until activated.
Using a denitrator the possibility Total S may contain HS due to sulfate reduction is considerably increased.
About the Total Toxicity of Total S present, I think an insignificant contributor to the Total S may be significant for the Total Toxicity.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,070
- Reaction score
- 64,516
Has someone claimed H2S will increase Total S?
HS does not increase Total S but the question is asked if high S may become a problem. As long one does not know what S represents one can not give a good answer.
If the tank stinks one has a much bigger problem as in a marine aquarium +90% will be HS; - 10% will be H2S.
When the water of a marine aquarium is saturated of H2S and stinks?
What is the most toxic, HS, or H2S?
If the water is saturated with H2S one will smell it.
HS may accumulate, not being part of the Total S in a water sample until activated.
Using a denitrator the possibility Total S may contain HS due to sulfate reduction is considerably increased.
About the Total Toxicity of Total S present, I think an insignificant contributor to the Total S may be significant for the Total Toxicity.
Not sure what the point of this discussion is.
In the context of sulfur being 700 ppm too high, no form of sulfide is responsible. Not S--, not H2S, not H2-.
Sulfate is the cause.
The connection is made between the Total S level and the use of an SD which is normal but more info of @ca1ore is needed.
A BADES application contributes to the presence of sulfate and Total S.
An increase of 600ppm S in a year's time is something I would like to follow up and clear up if possible.
Producing 600ppm Total S using a BADES reactor is not that difficult as it depends of the nitrate export rate, the consumption of elemental sulfur.
A BADES application contributes to the presence of sulfate and Total S.
An increase of 600ppm S in a year's time is something I would like to follow up and clear up if possible.
Producing 600ppm Total S using a BADES reactor is not that difficult as it depends of the nitrate export rate, the consumption of elemental sulfur.
Not sure what the point of this discussion is.
In the context of sulfur being 700 ppm too high, no form of sulfide is responsible. Not S--, not H2S, not H2-.
Sulfate is the cause.
What is the change the result may contain elemental sulfur particles as an SD is used?
Of course, one may expect it is mostly sulfate. As far as I know, sulfate is not toxic.
The question asked is if the Total S may be or may become a problem!
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,070
- Reaction score
- 64,516
What is the change the result may contain elemental sulfur particles as an SD is used?
Of course, one may expect it is mostly sulfate. As far as I know, sulfate is not toxic.
The question asked is if the Total S may be or may become a problem!
I do not understand what you are asking or saying.
Similar threads
- Replies
- 3
- Views
- 173
- Replies
- 3
- Views
- 146
- Replies
- 3
- Views
- 241
- Replies
- 4
- Views
- 163
- Replies
- 32
- Views
- 559


















