Hello reefers,
today, I received my first ICP-OES data. Before commenting on the obtained results, I would like to say that my livestock (including several acroporas) is doing great, and I dont observe any problems. Thus, my decision to perform ICP-OES (AF Labs) test was more curiosity-driven then done to search for causes of some issues. I also prefer to know about slowly developing issues before they cause obvious troubles. I use RO-DI unit with in-line TDS meter and the output is always 0-1 ppm. Now, I use Microbe-Lift Premium Reef salt, which is not so common; however, in following weeks, I will slowly transfer to RS Coral Pro as it better matches my desired parameters.
And now to the results. I dont copy the whole record as in most cases there are no significant issues. However:
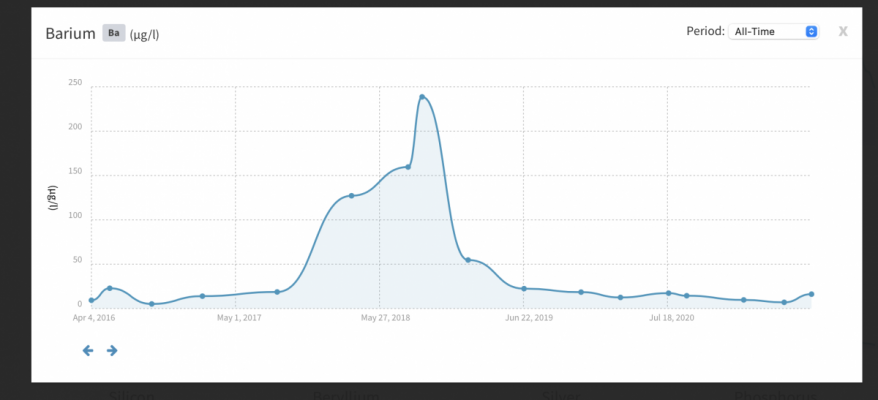
My Ba is 0.2230 mg/L, which seems to be incredibly high. Actualy, I suspect a kind of analysis error, improper sample handling or similar deviation (I work in a lab so I can imagine it).
Im quite confident that higher amounts of Ba should not have detrimental effects on livestock, but I find this concentration really to high too not cause any effect in long-term perspective.
This data was associated with:
Higher Al (0.0600 mg/L), and higher Zn (0.0377 mg/L). I actually dont consider these values too problematic, but I can imagine that long-term accumulation of these ions in organism could cause undesired effects. I have no idea what is the reason behind the high Ba/Al/Zn values. I didnt find any corrosion (I initally suspected the Radion XR15 G5 Pro mount, but it looks absolutely OK and it is not in contact with water at all), the tank room is free of deodorants, air freshener or whatever could contain Zn or Al...
Is there anybody with such experience? Any hints or discussion that could lead me to a solution of this issue might be helpful. Of course, I know that I can solve this by water change, etc., but I would rather prefer to find a culprit. Or do you think that I should forget it and let it be? Im not a fan of this idea.
Many thanks!
Zbynek
today, I received my first ICP-OES data. Before commenting on the obtained results, I would like to say that my livestock (including several acroporas) is doing great, and I dont observe any problems. Thus, my decision to perform ICP-OES (AF Labs) test was more curiosity-driven then done to search for causes of some issues. I also prefer to know about slowly developing issues before they cause obvious troubles. I use RO-DI unit with in-line TDS meter and the output is always 0-1 ppm. Now, I use Microbe-Lift Premium Reef salt, which is not so common; however, in following weeks, I will slowly transfer to RS Coral Pro as it better matches my desired parameters.
And now to the results. I dont copy the whole record as in most cases there are no significant issues. However:
My Ba is 0.2230 mg/L, which seems to be incredibly high. Actualy, I suspect a kind of analysis error, improper sample handling or similar deviation (I work in a lab so I can imagine it).
Im quite confident that higher amounts of Ba should not have detrimental effects on livestock, but I find this concentration really to high too not cause any effect in long-term perspective.
This data was associated with:
Higher Al (0.0600 mg/L), and higher Zn (0.0377 mg/L). I actually dont consider these values too problematic, but I can imagine that long-term accumulation of these ions in organism could cause undesired effects. I have no idea what is the reason behind the high Ba/Al/Zn values. I didnt find any corrosion (I initally suspected the Radion XR15 G5 Pro mount, but it looks absolutely OK and it is not in contact with water at all), the tank room is free of deodorants, air freshener or whatever could contain Zn or Al...
Is there anybody with such experience? Any hints or discussion that could lead me to a solution of this issue might be helpful. Of course, I know that I can solve this by water change, etc., but I would rather prefer to find a culprit. Or do you think that I should forget it and let it be? Im not a fan of this idea.
Many thanks!
Zbynek