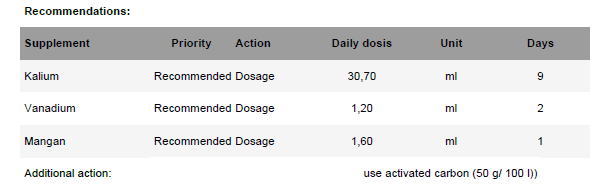
I received results from my first ATI ICP test, done out of curiosity rather than any particular problems other than an ongoing bubble algae issue that is coming under control. I'd be interested in feedback from those more experienced than I am with these tests.
Results are here: https://lab.atiaquaristik.com/publicAnalysis/230678. They should be public but I can post a screenshot if needed, and I attached a PDF of the results.
My 90-gallon tank is around 15 years old and I'm using Reef Crystals, with a 10 gallon (roughly 10%) water change every week or two. I'm doing bi-weekly dosing of Reef Builder, Reef Calcium, Reef Trace, and Reef Plus, with Red Sea magnesium as needed.
1. Asking for general opinions/advice - do any of the results jump out at anyone as a real problem?
2. Phosphate is 0.02 by ICP, 0.12 by Hanna ULR (tested at the same time I collected the ICP sample). The Hanna reading has been consistent at 0.12-0.15 over 5 weekly tests. I'm wondering whether I should throw out the Hanna tester? Or order the $15 calibration standards? I feed heavily but I have a lot of chaeto and have been using PhosGuard to keep that Hanna number from going any higher.
3. Iodine (183.9 ug/l) is 3x the "ideal value" of 61.88. Is this a problem? For a short time (a month, maybe), I was dosing Brightwell Iodion at half or less of their recommended dosing, but stopped a couple of months ago when I learned how inaccurate home iodine tests were. Is there any other potential ongoing source of iodine that could be contributing to this high value?
4. Copper is 3.30 ug/l - any issue with the copper level? I have a healthy population of snails and my emerald crabs are doing well, so I'm assuming this level isn't hazardous. I even had a bunch of baby snails sitting on a rock recently.
5. RODI water results were all non-detect except for nitrate at 2.0 mg/l. Salifert shows zero. I read in another thread that ICP isn't very accurate for nitrate? Is that correct, and could this be a false reading? In any case, my nitrates in the DT are good by both ICP and Salifert.
Also, just curious whether people use ICP as a sort of calibration of the other tests. For example, salinity is 33.61 with ATI, but it was 35 with my refractometer (which is calibrated to 35 ppt using Randy's recipe for a calibration solution). Would it make sense use a refractometer reading between 36 & 37 as being representative of a "true" 35 ppt?
Interesting (to me) observations: Magnesium testing was pretty close (1398 ICP vs. 1305 by Salifert), and so was nitrate (8.45 by ICP vs. 5 to 10 by Salifert. Alkalinity is 7.85 by ICP, 8.4 by Hanna; the Hanna is reading 7% higher; they claim a +/-5% accuracy, so this isn't too far off.
Thanks for any feedback!
Results are here: https://lab.atiaquaristik.com/publicAnalysis/230678. They should be public but I can post a screenshot if needed, and I attached a PDF of the results.
My 90-gallon tank is around 15 years old and I'm using Reef Crystals, with a 10 gallon (roughly 10%) water change every week or two. I'm doing bi-weekly dosing of Reef Builder, Reef Calcium, Reef Trace, and Reef Plus, with Red Sea magnesium as needed.
1. Asking for general opinions/advice - do any of the results jump out at anyone as a real problem?
2. Phosphate is 0.02 by ICP, 0.12 by Hanna ULR (tested at the same time I collected the ICP sample). The Hanna reading has been consistent at 0.12-0.15 over 5 weekly tests. I'm wondering whether I should throw out the Hanna tester? Or order the $15 calibration standards? I feed heavily but I have a lot of chaeto and have been using PhosGuard to keep that Hanna number from going any higher.
3. Iodine (183.9 ug/l) is 3x the "ideal value" of 61.88. Is this a problem? For a short time (a month, maybe), I was dosing Brightwell Iodion at half or less of their recommended dosing, but stopped a couple of months ago when I learned how inaccurate home iodine tests were. Is there any other potential ongoing source of iodine that could be contributing to this high value?
4. Copper is 3.30 ug/l - any issue with the copper level? I have a healthy population of snails and my emerald crabs are doing well, so I'm assuming this level isn't hazardous. I even had a bunch of baby snails sitting on a rock recently.
5. RODI water results were all non-detect except for nitrate at 2.0 mg/l. Salifert shows zero. I read in another thread that ICP isn't very accurate for nitrate? Is that correct, and could this be a false reading? In any case, my nitrates in the DT are good by both ICP and Salifert.
Also, just curious whether people use ICP as a sort of calibration of the other tests. For example, salinity is 33.61 with ATI, but it was 35 with my refractometer (which is calibrated to 35 ppt using Randy's recipe for a calibration solution). Would it make sense use a refractometer reading between 36 & 37 as being representative of a "true" 35 ppt?
Interesting (to me) observations: Magnesium testing was pretty close (1398 ICP vs. 1305 by Salifert), and so was nitrate (8.45 by ICP vs. 5 to 10 by Salifert. Alkalinity is 7.85 by ICP, 8.4 by Hanna; the Hanna is reading 7% higher; they claim a +/-5% accuracy, so this isn't too far off.
Thanks for any feedback!
Attachments
Last edited: